- Cyclooctene
-
cis- (top) and trans-Cyclooctene[1] 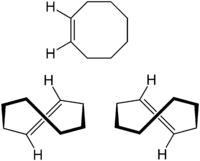 (E)-Cyclooctene
(E)-Cyclooctene
(Z)-CycloocteneOther namescis-Cyclooctene
trans-CycloocteneIdentifiers CAS number 931-87-3 PubChem 638079 ChemSpider 553642 Jmol-3D images Image 1 - C\1=C\CCCCCC/1
- InChI=1/C8H14/c1-2-4-6-8-7-5-3-1/h1-2H,3-8H2/b2-1-
Key: URYYVOIYTNXXBN-UPHRSURJBT
Properties Molecular formula C8H14 Molar mass 110.2 g mol−1 Density 0.846 g/mL Melting point -16 °C, 257 K, 3 °F
Boiling point 145-146 °C
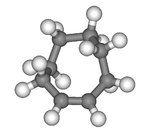
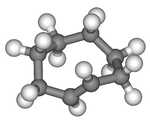
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Cyclooctene is a cycloalkene with an eight-membered ring. It is notable because it is the smallest cycloalkene that can exist as either the cis- or trans-isomer with the cis-isomer more common. Its most stable cis conformer is shaped like a crown ether with alternating equatorial and axial hydrogens very much like the chair conformation of cyclohexane; its most stable trans-conformer is shaped like the 8-carbon equivalent chair conformation of cyclohexane.
cis-Cyclooctene trans-Cyclooctene trans-Cyclooctene
trans-Cyclooctene is the smallest cycloalkene in which the trans-isomer is stable at room temperature (compared to cyclopropene for cis). This is because trans-cycloalkenes have a longer bridging distance between the two allylic carbons than their respective cis-cycloalkenes. As a result, eight carbons is the minimum ring size required to form a trans-cycloalkene without incurring severe angle strain which is the cause for the instability of smaller trans-rings. trans-Cycloheptene and trans-cyclohexene can exist, but they are very unstable at room temperature. trans-Cyclooctene exists in a helical conformation with the carbon chain lying above the double bond on one side and below it on the other, leading to chirality (as depicted to the right).
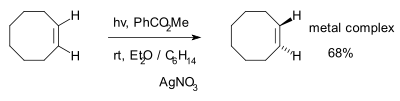
trans-Cyclooctene was first synthesized on a preparatory scale by Arthur C. Cope beginning from N,N,N-trimethylcyclooctylammonium iodide and using a Hofmann elimination.[2] Other methods exist where the trans-isomer is synthesized from the cis-isomer in several synthetic steps. In addition, a photochemical method exists for this conversion in just one step:
Although the cis-trans equilibrium is unfavorable, the reaction can be driven to completion by trapping of the trans-isomer by complexation with silver.[3]
References
- ^ cis-Cyclooctene at Sigma-Aldrich
- ^ Arthur C. Cope and Robert D. Bach "trans-Cyclooctene", Organic Syntheses, Coll. Vol. 5, p.315 (1973); Vol. 49, p.39 (1969)
- ^ A Photochemical Synthesis of Functionalized trans-Cyclooctenes Driven by Metal Complexation Maksim Royzen, Glenn P. A. Yap, and Joseph M. Fox J. AM. CHEM. SOC. 2008, 130, 3760-3761 doi:10.1021/ja8001919
Alkenes Dienes Cyclobutadiene · Cyclopentadiene · Cyclohexadiene (1,3-Cyclohexadiene · 1,4-Cyclohexadiene) · Cycloheptadiene · Cyclooctadiene (1,5-Cyclooctadiene)Categories:- Cycloalkenes
Wikimedia Foundation. 2010.