- Nylon 6
-
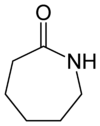
Nylon 6 or polycaprolactam is a polymer developed by Paul Schlack at IG Farben to reproduce the properties of nylon 6,6 without violating the patent on its production. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization. This makes it a special case in the comparison between condensation and addition polymers. Its competition with nylon 6,6 and the example it set have also shaped the economics of the synthetic fiber industry. It was given the trademark Perlon in 1952. It is a semicrystalline polyamide.
Contents
Synthesis
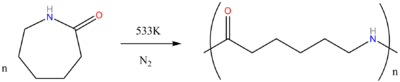
Nylon 6 begins as pure caprolactam. As caprolactam has 6 carbon atoms, it got the name Nylon-6.
When caprolactam is heated at about 533 K in an inert atmosphere of nitrogen for about 4-5 hours, the ring breaks and undergoes polymerization. Then the molten mass is passed through spinnerets to form fibres of Nylon 6.
During polymerization, the peptide bond within each caprolactam molecule is broken, with the active groups on each side re-forming two new bonds as the monomer becomes part of the polymer backbone. Unlike nylon 6,6, in which the direction of the amide bond reverses at each bond, all nylon 6 amide bonds lie in the same direction (see figure: note the N to C orientation of each amide bond). Nylon 6 therefore resembles natural polypeptides more closely; in fact, caprolactam would become an amino acid if it were hydrolyzed. This difference has little effect on the polymer's mechanical or chemical properties, but is sufficient to create a legal distinction.
 Nylon 6 (above) has a structure similar to Nylon 6,6 (below).
Nylon 6 (above) has a structure similar to Nylon 6,6 (below).Properties
Nylon 6 fibres are tough, possessing high tensile strength, as well as elasticity and lustre. They are wrinkle-proof and highly resistant to abrasion and chemicals such as acids and alkalis. The fibres can absorb up to 2.4% of water, although this lowers tensile strength.
Applications
Nylon 6 is used as thread in bristles for toothbrushes, surgical sutures, and strings for acoustic and classical musical instruments, including guitars, violins, violas, and cellos. It is also used in the manufacture of a large variety of threads, ropes, filaments, nets, and tire cords, as well as hosiery and knitted garments. It can also be used in gun frames, such as those used by Glock, which are made with a composite of Nylon 6 and other polymers.[1] It has the potential to be used as a technical nutrient.
References
External links
Categories:- Polyamides
- Plastics
- Synthetic fibers
Wikimedia Foundation. 2010.