- Oxaloacetic acid
-
Oxaloacetic acid 
 Oxobutanedioic acidOther namesOxaloacetic acid
Oxobutanedioic acidOther namesOxaloacetic acid
Oxalacetic acid
Oxosuccinic acidIdentifiers CAS number 328-42-7 
PubChem 970 ChemSpider 945 
EC number 206-329-8 ChEBI CHEBI:30744 
Jmol-3D images Image 1 - O=C(O)C(=O)CC(=O)O
Properties Molecular formula C4H4O5 Molar mass 132.07 g/mol Density ? g/cm3 Melting point 161 °C
Thermochemistry Std enthalpy of
formation ΔfHo298-943.21 kJ/mol Std enthalpy of
combustion ΔcHo298-1205.58 kJ/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oxaloacetic acid (also oxalacetic acid) is an organic compound with the chemical formula C4H4O5 or HOOC-(C=O)-(CH2)-COOH. It also has other names (see the table).
Its fully deprotonated derivative is the oxaloacetate anion, C4H2O52− or [(C=O)2(CH2)(C=O)]2−; this name is also used for esters that contain the divalent [-O(C=O)2(CH2)(C=O)O-] moiety. Loss of a single proton gives the acid's conjugate base, the anion hydrogenoxaloacetate anion H(C=O)2(CH2)(C=O)−.
Contents
Function
This four-carbon dicarboxylic acid is a protonated variant of oxaloacetate, which is an intermediate of the citric acid cycle[1] and gluconeogenesis. Oxaloacetate forms upon oxidation of L-malate, catalysed by malate dehydrogenase, and reacts with Acetyl-CoA to form citrate, catalysed by citrate synthase. It also forms in the mesophyll of plants by the condensation of CO2 with phosphoenolpyruvate, catalysed by PEP Carboxykinase. It can arise from pyruvate via an anaplerotic reaction. Oxaloacetate is also a potent inhibitor of Complex II.
Chemical properties
The enol forms of oxaloacetic acid are particularly stable, so much so that the two isomers have different melting points (152 °C cis, 184 °C trans).[clarification needed] The enol proton has a pKa value of 13.02.
Oxaloacetate is unstable in solution, decomposing to pyruvate by decarboxylation over a period of hours (room temperature) or days (0 °C).[citation needed] Refrigerated storage of the solid is therefore recommended.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[2]
Citric_acid_cycle edit
See also
References
- ^ "Citric Acid Cycle Reactions". http://www.elmhurst.edu/~chm/vchembook/611citricrx.html. Retrieved 2009-04-18.
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78". http://www.wikipathways.org/index.php/Pathway:WP78.
Citric Acid Cycle Metabolic Pathway Oxaloacetate Malate Fumarate Succinate Succinyl-CoA 
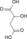
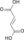

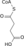
Acetyl-CoA NADH + H+ NAD+ H2O FADH2 FAD CoA + ATP(GTP) Pi + ADP(GDP) 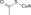
+ H2O 
NADH + H+ + CO2 CoA NAD+ 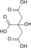
H2O 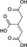
H2O 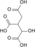
NAD(P)+ NAD(P)H + H+ 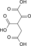
CO2 
Citrate cis-Aconitate Isocitrate Oxalosuccinate α-Ketoglutarate K→acetyl-CoA G G→pyruvate→citrateG→glutamate→
α-ketoglutarateotherα-Ketoisovaleric acid · Isobutyryl-CoA · Methacrylyl-CoA · 3-Hydroxyisobutyryl-CoA · 3-Hydroxyisobutyric acid · 2-Methyl-3-oxopropanoic acidG→fumarateG→oxaloacetatesee urea cycleOther biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i
This article about metabolism is a stub. You can help Wikipedia by expanding it.


