- Malate dehydrogenase
-
Malate dehydrogenase 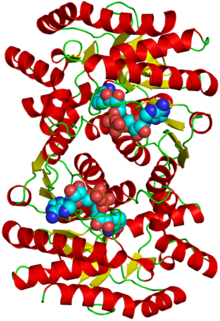
Structure of the protein with attached sugars Identifiers EC number 1.1.1.37 CAS number 9001-64-3 Databases IntEnz IntEnz view BRENDA BRENDA entry ExPASy NiceZyme view KEGG KEGG entry MetaCyc metabolic pathway PRIAM profile PDB structures RCSB PDB PDBe PDBsum Search PMC articles PubMed articles Malate dehydrogenase (EC 1.1.1.37) (MDH) is an enzyme in the citric acid cycle that catalyzes the conversion of malate into oxaloacetate (using NAD+) and vice versa (this is a reversible reaction). Malate dehydrogenase is not to be confused with malic enzyme, which catalyzes the conversion of malate to pyruvate, producing NADPH.
Malate dehydrogenase is also involved in gluconeogenesis, the synthesis of glucose from smaller molecules. Pyruvate in the mitochondria is acted upon by pyruvate carboxylase to form oxaloacetate, a citric acid cycle intermediate. In order to get the oxaloacetate out of the mitochondria, malate dehydrogenase reduces it to malate, and it then traverses the inner mitochondrial membrane. Once in the cytosol, the malate is oxidized back to oxaloacetate by cytosolic malate dehydrogenase. Finally, phosphoenol-pyruvate carboxy kinase (PEPCK) converts oxaloacetate to phosphoenol pyruvate.
Contents
Isozymes
Several isozymes of malate dehydrogenase exist, depending on where they are localized in the cell and their specific dependence on NAD+ or NADP+ (only in chloroplasts). There are two main isoforms in eukaryotic cells.[1] One is found in the mitochondrial matrix, participating as a key enzyme in the citric acid cycle that catalyzes the oxidation of malate. The other is found in the cytoplasm, assisting the malate-aspartate shuttle with exchanging reducing equivalents so that malate can pass through the mitochondrial membrane to be transformed into oxaloacetate for further cellular processes.[2]
Humans and most other mammals express the following two malate dehydrogenases:
malate dehydrogenase 1, NAD (soluble) Identifiers Symbol MDH1 Entrez 4190 HUGO 6970 OMIM 154200 RefSeq NM_005917 UniProt P40925 Other data EC number 1.1.1.37 Locus Chr. 2 p23 malate dehydrogenase 2, NAD (mitochondrial) Identifiers Symbol MDH2 Entrez 4191 HUGO 6971 OMIM 154100 RefSeq NM_005918 UniProt P40926 Other data EC number 1.1.1.37 Locus Chr. 7 cen-q22 Evolution and Structure
In most organisms, malate dehydrogenase exists as a homodimeric molecule and is closely related to lactate dehydrogenase in structure. It is a large protein molecule with subunits weighing between 30 and 35 kDa.[3] Based on the amino acid sequences, it seems that MDH has diverged into two main phylogenetic groups that closely resemble either mitochondrial isozymes or cytoplasmic/chloroplast isozymes.[4] Because the sequence identity of malate dehydrogenase in the mitochondria is more closely related to its prokaryotic ancestors in comparison to the cytoplasmic isozyme, the theory that mitochondria and chloroplasts were developed through endosymbiosis is plausible.[5] It is interesting to note that the amino acid sequences of archaeal MDH are more similar to that of LDH than that of MDH of other organisms. This indicates that there is a possible evolutionary linkage between lactate dehydrogenase and malate dehydrogenase.[6]
Each subunit of the malate dehydrogenase dimer has two distinct domains that vary in structure and functionality. A parallel β-sheet structure makes up the domain, while four β-sheets and one α-helix comprise the central NAD+ binding site. The subunits are held together through extensive hydrogen-bonding and hydrophobic interactions.[7]
Mechanism and Activity
The active site of malate dehydrogenase is a hydrophobic cavity within the protein complex that has specific binding sites for the substrate and its coenzyme, NAD+. In its active state, MDH undergoes a conformational change that encloses the substrate to minimize solvent exposure and to position key residues in closer proximity to the substrate.[4] The three residues in particular that comprise a catalytic triad are histidine (His-195), aspartate (Asp-168), both of which work together as a proton transfer system, and arginines (Arg-102, Arg-109, Arg-171), which secure the substrate.[8] Kinetic studies show that MDH enzymatic activity is ordered. NAD+/NADH is bound before the substrate.[9]
 Active site of malate dehydrogenase
Active site of malate dehydrogenase
Note: the structure of malate in the figure above is not correct. Malate should have four carbons, not six as the figure implies.Allosteric Regulation
Because malate dehydrogenase is closely tied to the citric acid cycle, regulation is highly dependent on TCA products. High malate concentrations stimulate MDH activity, and, in a converse manner, high oxaloacetate concentrations inhibit the enzyme.[10] Citrate can both allosterically activate and inhibit the enzymatic activity of MDH. It inhibits the oxidation of malate when there are low levels of malate and NAD+. However, in the presence of high levels of malate and NAD+, citrate can stimulate the production of oxaloacetate. It is believed that there is an allosteric regulatory site on the enzyme where citrate can bind to and drive the reaction equilibrium in either direction.[11]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[12]
References
- ^ Minárik P, Tomásková N, Kollárová M, Antalík M (September 2002). "Malate dehydrogenases--structure and function". Gen. Physiol. Biophys. 21 (3): 257–65. PMID 12537350.
- ^ Musrati RA, Kollárová M, Mernik N, Mikulásová D (September 1998). "Malate dehydrogenase: distribution, function and properties". Gen. Physiol. Biophys. 17 (3): 193–210. PMID 9834842.
- ^ Banaszak, LJ, Bradshaw RA (1975). "Malate dehydrogenase". In Boyer PD. The Enzymes. 11 (3rd ed.). New York: Academic Press. pp. 369–396.
- ^ a b Goward CR, Nicholls DJ (October 1994). "Malate dehydrogenase: a model for structure, evolution, and catalysis". Protein Sci. 3 (10): 1883–8. doi:10.1002/pro.5560031027. PMC 2142602. PMID 7849603. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2142602.
- ^ McAlister-Henn L (May 1988). "Evolutionary relationships among the malate dehydrogenases". Trends Biochem. Sci. 13 (5): 178–81. doi:10.1016/0968-0004(88)90146-6. PMID 3076279.
- ^ Cendrin F, Chroboczek J, Zaccai G, Eisenberg H, Mevarech M (April 1993). "Cloning, sequencing, and expression in Escherichia coli of the gene coding for malate dehydrogenase of the extremely halophilic archaebacterium Haloarcula marismortui". Biochemistry 32 (16): 4308–13. doi:10.1021/bi00067a020. PMID 8476859.
- ^ Hall MD, Levitt DG, Banaszak LJ (August 1992). "Crystal structure of Escherichia coli malate dehydrogenase. A complex of the apoenzyme and citrate at 1.87 A resolution". J. Mol. Biol. 226 (3): 867–82. doi:10.1016/0022-2836(92)90637-Y. PMID 1507230.
- ^ Lamzin VS, Dauter Z, Wilson KS (May 1994). "Dehydrogenation through the looking-glass". Nat. Struct. Biol. 1 (5): 281–2. doi:10.1038/nsb0594-281. PMID 7664032.
- ^ Shows TB, Chapman VM, Ruddle FH (December 1970). "Mitochondrial malate dehydrogenase and malic enzyme: Mendelian inherited electrophoretic variants in the mouse". Biochem. Genet. 4 (6): 707–18. doi:10.1007/BF00486384. PMID 5496232.
- ^ Mullinax TR, Mock JN, McEvily AJ, Harrison JH (November 1982). "Regulation of mitochondrial malate dehydrogenase. Evidence for an allosteric citrate-binding site". J. Biol. Chem. 257 (22): 13233–9. PMID 7142142.
- ^ Gelpí JL, Dordal A, Montserrat J, Mazo A, Cortés A (April 1992). "Kinetic studies of the regulation of mitochondrial malate dehydrogenase by citrate". Biochem. J. 283 ( Pt 1) (Pt 1): 289–97. PMC 1131027. PMID 1567375. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1131027.
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78". http://www.wikipathways.org/index.php/Pathway:WP78.
- Guha A, Englard S, Listowsky I (February 1968). "Beef heart malic dehydrogenases. VII. Reactivity of sulfhydryl groups and conformation of the supernatant enzyme". J. Biol. Chem. 243 (3): 609–15. PMID 5637713.
- McReynolds MS, Kitto GB (February 1970). "Purification and properties of Drosophila malate dehydrogenases". Biochim. Biophys. Acta 198 (2): 165–75. PMID 4313528.
- Wolfe RG, Nielands JB (July 1956). "Some molecular and kinetic properties of heart malic dehydrogenase". J. Biol. Chem. 221 (1): 61–9. PMID 13345798.
External links
Oxidoreductases: alcohol oxidoreductases (EC 1.1) 1.1.1: NAD/NADP acceptor Alcohol dehydrogenase · Aldo-keto reductase (1A1, 1B1, 1B10, 1C1, 1C3, 1C4, 7A2) · Aldose reductase · Carbohydrate dehydrogenases · Carnitine dehydrogenase · DXP reductoisomerase · Glucose-6-phosphate dehydrogenase · Glycerol-3-phosphate dehydrogenase · HMG-CoA reductase · 3-hydroxyacyl-CoA dehydrogenase · Beta-hydroxybutyryl-CoA dehydrogenase · Isocitrate dehydrogenase · IMP dehydrogenase · Β-Ketoacyl ACP reductase · Lactate dehydrogenase · Malate dehydrogenase · Phosphogluconate dehydrogenase · L-threonine dehydrogenase · L-xylulose reductase · Sorbitol dehydrogenase
Hydroxysteroid dehydrogenase: 3 Beta (3-beta-HSD, NSDHL) · 11 Beta (HSD11B1, HSD11B2) · 17 Beta1.1.2: cytochrome acceptor 1.1.3: oxygen acceptor 1.1.4: disulfide as acceptor 1.1.5: quinone/similar acceptor 1.1.99: other acceptors Metabolism: Citric acid cycle enzymes Cycle Citrate synthase · Aconitase · Isocitrate dehydrogenase · Oxoglutarate dehydrogenase · Succinyl CoA synthetase
Succinate dehydrogenase (SDHA) · Fumarase · Malate dehydrogenase and ETCAnaplerotic to acetyl-CoAPyruvate dehydrogenase complex (E1, E2, E3)
(regulated by Pyruvate dehydrogenase kinase and Pyruvate dehydrogenase phosphatase)to succinyl-CoAto oxaloacetateMitochondrial
electron transport chain/
oxidative phosphorylationPrimaryComplex I/NADH dehydrogenase · Complex II/Succinate dehydrogenase · Coenzyme Q · Complex III/Coenzyme Q - cytochrome c reductase · Cytochrome c · Complex IV/Cytochrome c oxidase
Coenzyme Q10 synthesis: COQ2 · COQ3 · COQ4 · COQ5 · COQ6 · COQ7 · COQ9 · COQ10A · COQ10B · PDSS1 · PDSS2OtherCategories:- Genes on chromosome 2
- Genes on chromosome 7
- EC 1.1.1
- Cellular respiration
Wikimedia Foundation. 2010.

