- Ornithine transcarbamylase
-
Ornithine transcarbamoylase (OTC) (also called ornithine carbamoyltransferase) is an enzyme that catalyzes the reaction between carbamoyl phosphate (CP) and ornithine (Orn) to form citrulline (Cit) and phosphate (Pi). In plants and microbes, OTC is involved in arginine (Arg) biosynthesis, whereas in mammals it is located in the mitochondria and is part of the urea cycle.
Contents
Structure
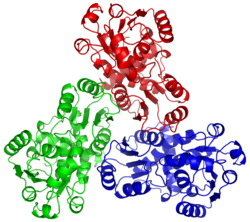
OTC is a trimer. The monomer unit has a CP-binding domain and an amino acid-binding domain. Each of the two discrete substrate-binding domains (SBDs) have an α/β topology with a central β-pleated sheet embedded in flanking α-helices.
The active sites are located at the interface between the protein monomers.
Function
 The reaction mechanism of OTC.
The reaction mechanism of OTC.
The side-chain amino group of Orn attacks the carbonyl carbon of CP nucleophilically, left, to form a tetrahedral transition state, middle. Charge rearrangement releases Cit and Pi, right. [1]Deficiency
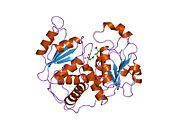
Main article: Ornithine transcarbamylase deficiency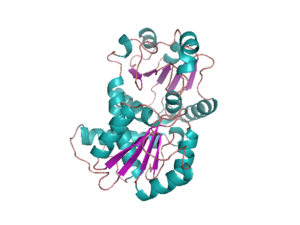 OTC monomer
OTC monomer
If a person is deficient in OTC, ammonia levels will build up, and this will cause neurological problems. Levels of the amino acids, glutamate, and alanine will be increased (as these are the amino acids that receive nitrogen from others).
Levels of urea cycle intermediates may be decreased, as carbamoyl phosphate cannot replenish the cycle. The carbamoyl phosphate instead goes into the uridine monophosphate synthetic pathway. Here orotic acid (one step of this alternative pathway) levels in the blood are increased.
A potential treatment for the high ammonia levels is to give sodium benzoate, which combines with glycine to produce hippurate, at the same time removing an ammonium group.
References
- a
 Mechanism of inactivation of ornithine transcarbamoylase by Ndelta -(N'-Sulfodiaminophosphinyl)-L-ornithine, a true transition state analogue? Crystal structure and implications for catalytic mechanism. J Biol Chem. 2000 Jun 30; 275: 20012-9; PubMed Free text
Mechanism of inactivation of ornithine transcarbamoylase by Ndelta -(N'-Sulfodiaminophosphinyl)-L-ornithine, a true transition state analogue? Crystal structure and implications for catalytic mechanism. J Biol Chem. 2000 Jun 30; 275: 20012-9; PubMed Free text
Further reading
- Tuchman M, Plante RJ (1995). "Mutations and polymorphisms in the human ornithine transcarbamylase gene: mutation update addendum". Hum. Mutat. 5 (4): 293–5. doi:10.1002/humu.1380050404. PMID 7627182.
- Tuchman M (1993). "Mutations and polymorphisms in the human ornithine transcarbamylase gene". Hum. Mutat. 2 (3): 174–8. doi:10.1002/humu.1380020304. PMID 8364586.
- Matsuda I, Tanase S (1997). "The ornithine transcarbamylase (OTC) gene: mutations in 50 Japanese families with OTC deficiency". Am. J. Med. Genet. 71 (4): 378–83. doi:10.1002/(SICI)1096-8628(19970905)71:4<378::AID-AJMG2>3.0.CO;2-Q. PMID 9286441.
- Wakabayashi Y (1999). "Tissue-selective expression of enzymes of arginine synthesis". Current opinion in clinical nutrition and metabolic care 1 (4): 335–9. PMID 10565370.
- Tuchman M, Jaleel N, Morizono H, et al. (2002). "Mutations and polymorphisms in the human ornithine transcarbamylase gene". Hum. Mutat. 19 (2): 93–107. doi:10.1002/humu.10035. PMID 11793468.
- Feldmann D, Rozet JM, Pelet A, et al. (1992). "Site specific screening for point mutations in ornithine transcarbamylase deficiency". J. Med. Genet. 29 (7): 471–5. PMC 1016021. PMID 1353535. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1016021.
- Tuchman M, Holzknecht RA, Gueron AB, et al. (1993). "Six new mutations in the ornithine transcarbamylase gene detected by single-strand conformational polymorphism". Pediatr. Res. 32 (5): 600–4. PMID 1480464.
- Dawson SJ, White LA (1992). "Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin". J. Infect. 24 (3): 317–20. doi:10.1016/S0163-4453(05)80037-4. PMID 1602151.
- Suess PJ, Tsai MY, Holzknecht RA, et al. (1992). "Screening for gene deletions and known mutations in 13 patients with ornithine transcarbamylase deficiency". Biochem. Med. Metab. Biol. 47 (3): 250–9. doi:10.1016/0885-4505(92)90033-U. PMID 1627356.
- Grompe M, Caskey CT, Fenwick RG (1991). "Improved molecular diagnostics for ornithine transcarbamylase deficiency". Am. J. Hum. Genet. 48 (2): 212–22. PMC 1683033. PMID 1671317. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1683033.
- Hentzen D, Pelet A, Feldman D, et al. (1992). "Fatal hyperammonemia resulting from a C-to-T mutation at a MspI site of the ornithine transcarbamylase gene". Hum. Genet. 88 (2): 153–6. PMID 1721894.
- Strautnieks S, Rutland P, Malcolm S (1992). "Arginine 109 to glutamine mutation in a girl with ornithine carbamoyl transferase deficiency". J. Med. Genet. 28 (12): 871–4. doi:10.1136/jmg.28.12.871. PMC 1017166. PMID 1757964. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1017166.
- Carstens RP, Fenton WA, Rosenberg LR (1991). "Identification of RNA splicing errors resulting in human ornithine transcarbamylase deficiency". Am. J. Hum. Genet. 48 (6): 1105–14. PMC 1683104. PMID 2035531. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1683104.
- Hata A, Matsuura T, Setoyama C, et al. (1991). "A novel missense mutation in exon 8 of the ornithine transcarbamylase gene in two unrelated male patients with mild ornithine transcarbamylase deficiency". Hum. Genet. 87 (1): 28–32. doi:10.1007/BF01213087. PMID 2037279.
- Legius E, Baten E, Stul M, et al. (1990). "Sporadic late onset ornithine transcarbamylase deficiency in a boy with somatic mosaicism for an intragenic deletion". Clin. Genet. 38 (2): 155–9. doi:10.1111/j.1399-0004.1990.tb03565.x. PMID 2208768.
- Finkelstein JE, Francomano CA, Brusilow SW, Traystman MD (1990). "Use of denaturing gradient gel electrophoresis for detection of mutation and prospective diagnosis in late onset ornithine transcarbamylase deficiency". Genomics 7 (2): 167–72. doi:10.1016/0888-7543(90)90537-5. PMID 2347583.
- Grompe M, Muzny DM, Caskey CT (1989). "Scanning detection of mutations in human ornithine transcarbamoylase by chemical mismatch cleavage". Proc. Natl. Acad. Sci. U.S.A. 86 (15): 5888–92. doi:10.1073/pnas.86.15.5888. PMC 297736. PMID 2474822. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=297736.
- Lee JT, Nussbaum RL (1990). "An arginine to glutamine mutation in residue 109 of human ornithine transcarbamylase completely abolishes enzymatic activity in Cos1 cells". J. Clin. Invest. 84 (6): 1762–6. doi:10.1172/JCI114360. PMC 304053. PMID 2556444. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=304053.
- Chu TW, Eftime R, Sztul E, Strauss AW (1989). "Synthetic transit peptides inhibit import and processing of mitochondrial precursor proteins". J. Biol. Chem. 264 (16): 9552–8. PMID 2722850.
- Hata A, Setoyama C, Shimada K, et al. (1989). "Ornithine transcarbamylase deficiency resulting from a C-to-T substitution in exon 5 of the ornithine transcarbamylase gene". Am. J. Hum. Genet. 45 (1): 123–7. PMC 1683378. PMID 2741942. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1683378.
External links
PDB gallery 1c9y: HUMAN ORNITHINE TRANSCARBAMYLASE: CRYSTALLOGRAPHIC INSIGHTS INTO SUBSTRATE RECOGNITION AND CATALYTIC MECHANISM1ep9: HUMAN ORNITHINE TRANSCARBAMYLASE: CRYSTALLOGRAPHIC INSIGHTS INTO SUBSTRATE RECOGNITION AND CONFORMATIONAL CHANGE1fb5: LOW RESOLUTION STRUCTURE OF OVINE ORNITHINE TRANSCARBMOYLASE IN THE UNLIGANDED STATE1fvo: CRYSTAL STRUCTURE OF HUMAN ORNITHINE TRANSCARBAMYLASE COMPLEXED WITH CARBAMOYL PHOSPHATE1oth: CRYSTAL STRUCTURE OF HUMAN ORNITHINE TRANSCARBAMOYLASE COMPLEXED WITH N-PHOSPHONACETYL-L-ORNITHINETransferase: one carbon transferases (EC 2.1) 2.1.1: Methyl- N-O-Other2.1.2: Hydroxymethyl-,
Formyl- and RelatedHydroxymethyltransferaseFormyltransferaseOther2.1.3: Carboxy- and Carbamoyl CarboxyCarbamoyl2.1.4: Amidino Arginine:glycine amidinotransferaseMain cycle mitochondrial matrix: Carbamoyl phosphate synthetase I · Ornithine transcarbamylase
cytosol: Argininosuccinate synthetase · Argininosuccinate lyase · ArginaseRegulatory/transport Mitochondrial proteins Outer membrane Intermembrane space Inner membrane oxidative phosphorylation (Coenzyme Q - cytochrome c reductase, Cytochrome c, NADH dehydrogenase, Succinate dehydrogenase)
pyrimidine metabolism (Dihydroorotate dehydrogenase)
mitochondrial shuttle (Malate-aspartate shuttle, Glycerol phosphate shuttle)
other (Glutamate aspartate transporter, Glycerol-3-phosphate dehydrogenase, ATP synthase, Carnitine palmitoyltransferase II, Uncoupling protein)Matrix citric acid cycle (Citrate synthase, Aconitase, Isocitrate dehydrogenase, Oxoglutarate dehydrogenase, Succinyl coenzyme A synthetase, Fumarase, Malate dehydrogenase)
anaplerotic reactions (Aspartate transaminase, Glutamate dehydrogenase, Pyruvate dehydrogenase complex)
urea cycle (Carbamoyl phosphate synthetase I, Ornithine transcarbamylase, N-Acetylglutamate synthase)
alcohol metabolism (ALDH2)
PMPCBOther/to be sorted Mitochondrial DNA Complex I (MT-ND1, MT-ND2, MT-ND3, MT-ND4, MT-ND4L, MT-ND5, MT-ND6) - Complex III (MT-CYB) - Complex IV (MT-CO1, MT-CO2, MT-CO3)
ATP synthase (MT-ATP6, MT-ATP8)
tRNA (MT-TA, MT-TC, MT-TD, MT-TE, MT-TF, MT-TG, MT-TH, MT-TI, MT-TK, MT-TL1, MT-TL2, MT-TM, MT-TN, MT-TP, MT-TQ, MT-TR, MT-TS1, MT-TS2, MT-TT, MT-TV, MT-TW, MT-TY)Categories:- Human proteins
- EC 2.1.3
- Urea cycle
- a
Wikimedia Foundation. 2010.