- Cholesterol side-chain cleavage enzyme
-
cholesterol monooxygenase (side-chain-cleaving) Identifiers EC number 1.14.15.6 CAS number 37292-81-2 Databases IntEnz IntEnz view BRENDA BRENDA entry ExPASy NiceZyme view KEGG KEGG entry MetaCyc metabolic pathway PRIAM profile PDB structures RCSB PDB PDBe PDBsum Gene Ontology AmiGO / EGO Search PMC articles PubMed articles Cytochrome P450, family 11, subfamily A, polypeptide 1 (P45011a1), often referred to as P450scc (or 20,22-desmolase), is a mitochondrial enzyme associated with the conversion of cholesterol to pregnenolone. The gene name is CYP11A1.[1] The term "desmolase" is becoming outdated and the enzyme is now commonly referred to as P450scc, where "scc" refers to side-chain cleavage.
The protein is a member of the cytochrome P450 superfamily of enzymes. It catalyzes the first step in all steroid hormone production -- the conversion of cholesterol to pregnenolone, the first steroid formed:
- cholesterol + reduced adrenal ferredoxin + O2
 pregnenolone + 4-methylpentanal + oxidized adrenal ferredoxin + H2O
pregnenolone + 4-methylpentanal + oxidized adrenal ferredoxin + H2O
The protein resides on the inner mitochondrial membrane, facing the interior (matrix).[2] The presence of this enzyme defines if a cell is steroidogenic. Consequently, P450scc is found in all steroid-producing cell types such as theca cells and luteal cells in the ovary, Leydig cells in the testis, and cell types in the adrenal cortex.
Contents
Nomenclature
The systematic name of this enzyme class is cholesterol,reduced-adrenal-ferredoxin:oxygen oxidoreductase (side-chain-cleaving). Other names in common use include:
- C27-side-chain cleavage enzyme,
- cholesterol 20-22-desmolase,
- cholesterol C20-22 desmolase,
- cholesterol desmolase,
- cholesterol side-chain cleavage enzyme,
- cholesterol side-chain-cleaving enzyme,
- cytochrome P-450scc,
- desmolase, steroid 20-22,
- enzymes, cholesterol side-chain-cleaving,
- steroid 20-22 desmolase, and
- steroid 20-22-lyase.
Mechanism of action
P450scc catalyzes the conversion of cholesterol to pregnenolone in three monooxygenase reactions. They involve 2 hydroxylations of the cholesterol side-chain, which generate, first, 22R-hydroxycholesterol and then 20alpha,22R-dihydroxycholesterol. The final step cleaves the bond between carbons 20 and 22, resulting in the production of pregnenolone and isocaproic acid.
Each step requires 2 electrons (reducing equivalents). These are provided by 2 cofactors: adrenodoxin reductase and adrenodoxin, which shuttles electrons from the latter protein to P450scc. All three proteins together constitute the cholesterol side-chain cleavage complex.
The activity of P450scc is blocked by the drug aminoglutethimide.
Regulation
The expression of CYP11A1 in the adrenal and gonads is principally regulated by angiotensin II and two pituitary hormones, LH and ACTH [3]. They increase CYP11A1 gene expression through transcription factors such as steroidogenic factor 1 (SF-1), by the α isoform of activating protein 2 (AP-2) in the human, and many others [3][4]. The production of this enzyme is inhibited notably by the nuclear receptor DAX-1 [3].
P450scc is always active, however its activity is limited by the supply of cholesterol in the inner membrane. The supplying of cholesterol to this membrane (from the outer mitochondrial membrane) is thus considered the true rate-limiting step in steroid production. This step is primarily mediated by the steroidogenic acute regulatory protein (StAR or STARD1). Upon stimulation of a cell to make steroid, the amount of StAR available to transfer cholesterol to the inner membrane limits how fast the reaction can go (the acute phase). With prolonged (chronic) stimulation, it is thought that cholesterol supply becomes no longer an issue and that the capacity of the system to make steroid (i.e., level of P450scc in the mitochondria) is now more important.
Pathology
Mutations in the CYP11A1 gene result in a steroid hormone deficiency, causing a minority of cases of the rare and potentially fatal condition, lipoid congenital adrenal hyperplasia[5][6][7].
References
- ^ "Entrez Gene: CYP11A1 cytochrome P450, family 11, subfamily A, polypeptide 1". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1583.
- ^ Farkash Y, Timberg R, Orly J (April 1986). "Preparation of antiserum to rat cytochrome P-450 cholesterol side chain cleavage, and its use for ultrastructural localization of the immunoreactive enzyme by protein A-gold technique". Endocrinology 118 (4): 1353–65. doi:10.1210/endo-118-4-1353. PMID 3948785.
- ^ a b c Lavoie HA, King SR (2009). "Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B.". Exp. Biol. Med. (Maywood) 234 (8): 880–907. doi:10.3181/0903-MR-97. PMID 19491374.
- ^ Guo IC, Shih MC, Lan HC, et al. (2007). "Transcriptional regulation of human CYP11A1 in gonads and adrenals.". J. Biomed. Sci. 14 (4): 509–15. doi:10.1007/s11373-007-9177-z. PMID 17594537.
- ^ Bhangoo A, Anhalt H, Ten S, King SR (March 2006). "Phenotypic variations in lipoid congenital adrenal hyperplasia.". Pediatr. Endocrinol. Rev. 3 (3): 258–71. PMID 16639391.
- ^ al Kandari H, Katsumata N, Alexander S, Rasoul MA (2006). "Homozygous mutation of P450 side-chain cleavage enzyme gene (CYP11A1) in 46,XY patient with adrenal insufficiency, complete sex reversal, and agenesis of corpus callosum.". J. Clin. Endocr. Metab. 91 (8): 2821–6. doi:10.1210/jc.2005-2230. PMID 16705068.
- ^ Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, Miller WL (March 2008). "Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side-chain cleavage enzyme, P450scc". J. Clin. Endocrinol. Metab. 93 (3): 696–702. doi:10.1210/jc.2007-2330. PMC 2266942. PMID 18182448. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=18182448.
Further reading
- Hanukoglu I, Jefcoate CR (1980). "Mitochondrial cytochrome P-450sec. Mechanism of electron transport by adrenodoxin". J. Biol. Chem. 255 (7): 3057–61. PMID 6766943.
- Hanukoglu I, Spitsberg V, Bumpus JA, Dus KM, Jefcoate CR (1981). "Adrenal mitochondrial cytochrome P-450scc. Cholesterol and adrenodoxin interactions at equilibrium and during turnover". J. Biol. Chem. 256 (9): 4321–8. PMID 7217084.
- Helmberg A (1993). "Twin genes and endocrine disease: CYP21 and CYP11B genes.". Acta Endocrinol. 129 (2): 97–108. PMID 8372604.
- Papadopoulos V, Amri H, Boujrad N, et al. (1997). "Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis.". Steroids 62 (1): 21–8. doi:10.1016/S0039-128X(96)00154-7. PMID 9029710.
- Stocco DM (2000). "Intramitochondrial cholesterol transfer.". Biochim. Biophys. Acta 1486 (1): 184–97. PMID 10856721.
- Kristensen VN, Kure EH, Erikstein B, et al. (2001). "Genetic susceptibility and environmental estrogen-like compounds.". Mutat. Res. 482 (1-2): 77–82. doi:10.1016/S0027-5107(01)00212-3. PMID 11535251.
- Strauss JF (2004). "Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome.". Ann. N. Y. Acad. Sci. 997 (1): 42–8. doi:10.1196/annals.1290.005. PMID 14644808.
- Guo IC, Shih MC, Lan HC, et al. (2007). "Transcriptional regulation of human CYP11A1 in gonads and adrenals.". J. Biomed. Sci. 14 (4): 509–15. doi:10.1007/s11373-007-9177-z. PMID 17594537.
- Wada A, Waterman MR (1992). "Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding.". J. Biol. Chem. 267 (32): 22877–82. PMID 1429635.
- Hu MC, Guo IC, Lin JH, Chung BC (1991). "Regulated expression of cytochrome P-450scc (cholesterol-side-chain cleavage enzyme) in cultured cell lines detected by antibody against bacterially expressed human protein.". Biochem. J.. 274 ( Pt 3): 813–7. PMC 1149983. PMID 1849407. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1149983.
- Sparkes RS, Klisak I, Miller WL (1991). "Regional mapping of genes encoding human steroidogenic enzymes: P450scc to 15q23-q24, adrenodoxin to 11q22; adrenodoxin reductase to 17q24-q25; and P450c17 to 10q24-q25.". DNA Cell Biol. 10 (5): 359–65. doi:10.1089/dna.1991.10.359. PMID 1863359.
- Coghlan VM, Vickery LE (1991). "Site-specific mutations in human ferredoxin that affect binding to ferredoxin reductase and cytochrome P450scc.". J. Biol. Chem. 266 (28): 18606–12. PMID 1917982.
- Matteson KJ, Chung BC, Urdea MS, Miller WL (1986). "Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes.". Endocrinology 118 (4): 1296–305. doi:10.1210/endo-118-4-1296. PMID 2419119.
- Chung BC, Matteson KJ, Voutilainen R, et al. (1987). "Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta.". Proc. Natl. Acad. Sci. U.S.A. 83 (23): 8962–6. doi:10.1073/pnas.83.23.8962. PMC 387054. PMID 3024157. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=387054.
- Morohashi K, Sogawa K, Omura T, Fujii-Kuriyama Y (1987). "Gene structure of human cytochrome P-450(SCC), cholesterol desmolase.". J. Biochem. 101 (4): 879–87. PMID 3038854.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides.". Gene 138 (1-2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Gharani N, Waterworth DM, Batty S, et al. (1997). "Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism.". Hum. Mol. Genet. 6 (3): 397–402. doi:10.1093/hmg/6.3.397. PMID 9147642.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library.". Gene 200 (1-2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Hukkanen J, Mäntylä M, Kangas L, et al. (1998). "Expression of cytochrome P450 genes encoding enzymes active in the metabolism of tamoxifen in human uterine endometrium.". Pharmacol. Toxicol. 82 (2): 93–7. doi:10.1111/j.1600-0773.1998.tb01404.x. PMID 9498238.
- Zhou Z, Shackleton CH, Pahwa S, et al. (1998). "Prominent sex steroid metabolism in human lymphocytes.". Mol. Cell. Endocrinol. 138 (1-2): 61–9. doi:10.1016/S0303-7207(98)00052-5. PMID 9685215.
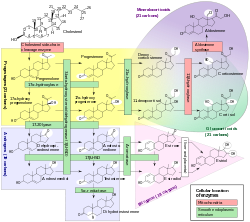
Steroid hormone synthesis
Additional images
External links
See also
- Cytochrome P450 oxidase
Mevalonate pathway To HMG-CoATo DMAPPGeranyl-To cholesterol To lanosterol7-Dehydrocholesterol pathDesmosterol pathTo Bile acids Steroidogenesis To pregnenoloneSide-chain cleavageTo sex hormonesTo androgensTo estrogensOther/ungroupedCytochromes, oxygenases: cytochrome P450 (EC 1.14) CYP1 CYP2 CYP3 (CYP3A) CYP4 CYP5-20 CYP21-51 Oxidoreductases: dioxygenases, including steroid hydroxylases (EC 1.14) 1.14.11: 2-oxoglutarate 1.14.13: NADH or NADPH Flavin-containing monooxygenase (FMO1, FMO2, FMO3, FMO4, FMO5) - Nitric oxide synthase (NOS1, NOS2, NOS3) - Cholesterol 7 alpha-hydroxylase - Methane monooxygenase - 3A4 - Lanosterol 14 alpha-demethylase1.14.14: reduced flavin or flavoprotein 1.14.15: reduced iron-sulfur protein 1.14.16: reduced pteridine (BH4 dependent) 1.14.17: reduced ascorbate 1.14.18-19: other 1.14.99 - miscellaneous Photosynthesis Dehydrogenase Other CAD (Carbamoyl phosphate synthase II, Aspartate carbamoyltransferase, Dihydroorotase) · Cholesterol side-chain cleavage enzyme · Cytochrome b6f complex · Electron transport chain · Fatty acid synthetase complex · Glycine decarboxylase complex · Mitochondrial trifunctional protein (HADHA, HADHB) · Phosphoenolpyruvate sugar phosphotransferase system · Polyketide synthase · Sucrase-isomaltase complex · Tryptophan synthaseMitochondrial proteins Outer membrane Intermembrane space Inner membrane oxidative phosphorylation (Coenzyme Q - cytochrome c reductase, Cytochrome c, NADH dehydrogenase, Succinate dehydrogenase)
pyrimidine metabolism (Dihydroorotate dehydrogenase)
mitochondrial shuttle (Malate-aspartate shuttle, Glycerol phosphate shuttle)
other (Glutamate aspartate transporter, Glycerol-3-phosphate dehydrogenase, ATP synthase, Carnitine palmitoyltransferase II, Uncoupling protein)Matrix citric acid cycle (Citrate synthase, Aconitase, Isocitrate dehydrogenase, Oxoglutarate dehydrogenase, Succinyl coenzyme A synthetase, Fumarase, Malate dehydrogenase)
anaplerotic reactions (Aspartate transaminase, Glutamate dehydrogenase, Pyruvate dehydrogenase complex)
urea cycle (Carbamoyl phosphate synthetase I, Ornithine transcarbamylase, N-Acetylglutamate synthase)
alcohol metabolism (ALDH2)
PMPCBOther/to be sorted steroidogenesis (Cholesterol side-chain cleavage enzyme, Steroid 11-beta-hydroxylase, Aldosterone synthase), Frataxin
Mitochondrial membrane transport protein (Mitochondrial permeability transition pore, Mitochondrial carrier)Mitochondrial DNA Complex I (MT-ND1, MT-ND2, MT-ND3, MT-ND4, MT-ND4L, MT-ND5, MT-ND6) - Complex III (MT-CYB) - Complex IV (MT-CO1, MT-CO2, MT-CO3)
ATP synthase (MT-ATP6, MT-ATP8)
tRNA (MT-TA, MT-TC, MT-TD, MT-TE, MT-TF, MT-TG, MT-TH, MT-TI, MT-TK, MT-TL1, MT-TL2, MT-TM, MT-TN, MT-TP, MT-TQ, MT-TR, MT-TS1, MT-TS2, MT-TT, MT-TV, MT-TW, MT-TY)Categories:- Human proteins
- Chromosome 15 gene stubs
- Cytochrome P450
- cholesterol + reduced adrenal ferredoxin + O2
Wikimedia Foundation. 2010.