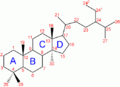
- CYP17A1
-
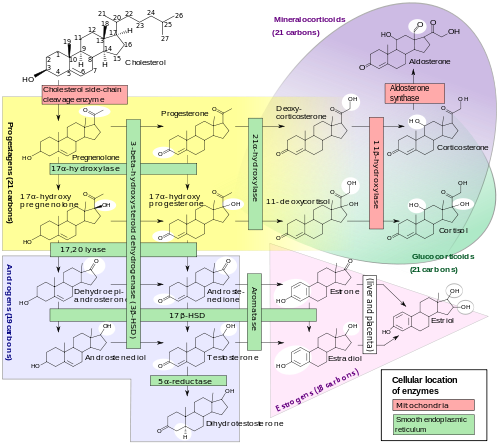
CYP17A1 also known as cytochrome P450 17A1, or steroid 17-alpha-monooxygenase, or 17α-hydroxylase/17,20 lyase/17,20 desmolase[1] is a cytochrome P450 enzyme that acts upon pregnenolone and progesterone to add a hydroxyl (-OH) group at carbon 17 of the steroid D ring (the hydroxylase activity), or acts upon 17-hydroxyprogesterone and 17-hydroxypregnenolone to split the side-chain off the steroid nucleus (the lyase activity).
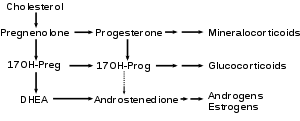 17α-hydroxylase converts pregnenolone and progesterone to their 17-hydroxy forms, and converts 17-hydroxypregnenolone and 17-hydroxyprogesterone to DHEA and androstenedione, respectively. It corresponds to the downward arrows in this reaction scheme.
17α-hydroxylase converts pregnenolone and progesterone to their 17-hydroxy forms, and converts 17-hydroxypregnenolone and 17-hydroxyprogesterone to DHEA and androstenedione, respectively. It corresponds to the downward arrows in this reaction scheme.
This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases that catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids, and other lipids. This protein localizes to the endoplasmic reticulum. It has both 17alpha-hydroxylase and 17,20-lyase activities, and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. Mutations in this gene are associated with 17 alpha-hydroxylase deficiency, 17 alpha-hydroxylase/17,20-lyase deficiency, pseudohermaphroditism, and adrenal hyperplasia.[2]
Contents
Steroidogenesis
Additional images
See also
References
- ^ Medical Physiology, Boron & Boulpaep, ISBN 1-4160-2328-3, Elsevier Saunders 2005. Updated edition. Page 1180.
- ^ "Entrez Gene: CYP17A1 cytochrome P450, family 17, subfamily A, polypeptide 1". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1586.
Further reading
- Miura K, Yasuda K, Yanase T et al. (1996). "Mutation of cytochrome P-45017 alpha gene (CYP17) in a Japanese patient previously reported as having glucocorticoid-responsive hyperaldosteronism: with a review of Japanese patients with mutations of CYP17". J. Clin. Endocrinol. Metab. 81 (10): 3797–801. doi:10.1210/jc.81.10.3797. PMID 8855840.
- Miller WL, Geller DH, Auchus RJ (1999). "The molecular basis of isolated 17,20 lyase deficiency". Endocr. Res. 24 (3–4): 817–25. doi:10.3109/07435809809032692. PMID 9888582.
- Strauss JF (2004). "Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome". Ann. N. Y. Acad. Sci. 997: 42–8. doi:10.1196/annals.1290.005. PMID 14644808.
- Haider S, Patel J, Poojari C, Neidle S (2010). "Molecular modeling on inhibitor complexes and active-site dynamics of cytochrome P450 C17, a target for prostate cancer therapy". J. Mol Biol 400 (5): 1078–098. doi:10.1016/j.jmb.2010.05.069. PMID 20595043.
External links
Oxidoreductases: dioxygenases, including steroid hydroxylases (EC 1.14) 1.14.11: 2-oxoglutarate 1.14.13: NADH or NADPH Flavin-containing monooxygenase (FMO1, FMO2, FMO3, FMO4, FMO5) - Nitric oxide synthase (NOS1, NOS2, NOS3) - Cholesterol 7 alpha-hydroxylase - Methane monooxygenase - 3A4 - Lanosterol 14 alpha-demethylase1.14.14: reduced flavin or flavoprotein 1.14.15: reduced iron-sulfur protein 1.14.16: reduced pteridine (BH4 dependent) 1.14.17: reduced ascorbate 1.14.18-19: other 1.14.99 - miscellaneous Mevalonate pathway To HMG-CoAAcetyl-Coenzyme A acetyltransferase · HMG-CoA synthase (regulated step)To DMAPPGeranyl-To cholesterol To lanosterol7-Dehydrocholesterol pathDesmosterol pathTo Bile acids Steroidogenesis To pregnenolonecortisol/cortisone: 17α-hydroxylase · 11β dehydrogenase (HSD11B1, HSD11B2)
both: 3β dehydrogenase · 21α-hydroxylase · 11β-hydroxylaseTo sex hormonesTo androgensTo estrogensOther/ungroupedCytochromes, oxygenases: cytochrome P450 (EC 1.14) CYP1 CYP2 CYP3 (CYP3A) CYP4 CYP5-20 CYP21-51 
This enzyme-related article is a stub. You can help Wikipedia by expanding it.