- Methionine synthase
-
Methionine synthase also known as MS, MeSe, MetH is an enzyme that in humans is encoded by the MTR gene (5-methyltetrahydrofolate-homocysteine methyltransferase).[1][2] This enzyme is responsible for the regeneration of methionine from homocysteine. Methionine synthase forms part of the S-adenosylmethionine (SAMe) biosynthesis and regeneration cycle.[3]
Contents
Function
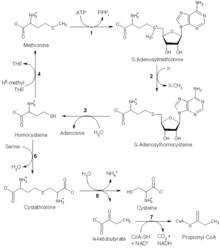
Methionine synthase catalyzes the final step in the regeneration of methionine from homocysteine.
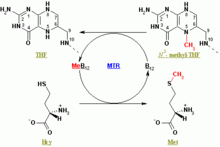
Methionine synthase contains the cofactor methylcobalamin (MeB12) and uses the substrates N5-methyl-tetrahydrofolate (N5-mTHF) and homocysteine.
The enzyme works in two steps in a ping-pong reaction. First, methylcobalamin is formed by a methyl group transfer from N5-mTHF with formation of MeB12 and tetrahydrofolate (THF). In the second step, MeB12 transfers this methyl group to homocysteine, regenerating the cofactor cobalamin and releasing the product methionine.
Methionine synthase is the only mammalian enzyme that metabolizes 5-mTHF to regenerate the active cofactor THF. Deficiency in methionine synthase function may be due to genetic mutations, reduced levels of its cobalamin cofactor (vitamin B12), or decreased levels of the enzyme (methionine synthase) reductase (required for the sustained activity of methionine synthase).
Clinical significance
Mutations in the MTR gene have been identified as the underlying cause of methylcobalamin deficiency complementation group G, or methylcobalamin deficiency cblG-type.[1] The consequence of reduced methionine synthase activity is megaloblastic anemia.
Genetics
Several polymorphisms in the MTR gene have been identified.[citation needed]
- 2756A→G (Asp919Gly)
See also
- Methyltransferase
- Arakawa's syndrome II
- 5-Methyltetrahydrofolate (5-Me-THF, 5-Me-H4F)
References
- ^ a b "MTR 5-methyltetrahydrofolate-homocysteine methyltransferase (Homo sapiens)". Entrez. 19 May 2009. http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4548. Retrieved 24 May 2009.
- ^ Li YN, Gulati S, Baker PJ, Brody LC, Banerjee R, Kruger WD (December 1996). "Cloning, mapping and RNA analysis of the human methionine synthase gene". Hum. Mol. Genet. 5 (12): 1851–8. doi:10.1093/hmg/5.12.1851. PMID 8968735.
- ^ Banerjee RV, Matthews RG (March 1990). "Cobalamin-dependent methionine synthase". FASEB J. 4 (5): 1450–9. PMID 2407589. http://www.fasebj.org/cgi/reprint/4/5/1450.pdf.
Further reading
- Banerjee RV, Matthews RG (1990). "Cobalamin-dependent methionine synthase.". FASEB J. 4 (5): 1450–9. PMID 2407589.
- Ludwig ML, Matthews RG (1997). "Structure-based perspectives on B12-dependent enzymes.". Annu. Rev. Biochem. 66: 269–313. doi:10.1146/annurev.biochem.66.1.269. PMID 9242908.
- Matthews RG, Sheppard C, Goulding C (1998). "Methylenetetrahydrofolate reductase and methionine synthase: biochemistry and molecular biology.". Eur. J. Pediatr. 157 Suppl 2: S54–9. doi:10.1007/PL00014305. PMID 9587027.
- Garovic-Kocic V, Rosenblatt DS (1992). "Methionine auxotrophy in inborn errors of cobalamin metabolism.". Clinical and investigative medicine. Médecine clinique et experimentale 15 (4): 395–400. PMID 1516297.
- O'Connor DL, Moriarty P, Picciano MF (1992). "The impact of iron deficiency on the flux of folates within the mammary gland.". International journal for vitamin and nutrition research. Internationale Zeitschrift für Vitamin- und Ernährungsforschung. Journal international de vitaminologie et de nutrition 62 (2): 173–80. PMID 1517041.
- Everman BW, Koblin DD (1992). "Aging, chronic administration of ethanol, and acute exposure to nitrous oxide: effects on vitamin B12 and folate status in rats.". Mech. Ageing Dev. 62 (3): 229–43. doi:10.1016/0047-6374(92)90109-Q. PMID 1583909.
- Vassiliadis A, Rosenblatt DS, Cooper BA, Bergeron JJ (1991). "Lysosomal cobalamin accumulation in fibroblasts from a patient with an inborn error of cobalamin metabolism (cblF complementation group): visualization by electron microscope radioautography.". Exp. Cell Res. 195 (2): 295–302. doi:10.1016/0014-4827(91)90376-6. PMID 2070814.
- Li YN, Gulati S, Baker PJ, et al. (1997). "Cloning, mapping and RNA analysis of the human methionine synthase gene.". Hum. Mol. Genet. 5 (12): 1851–8. doi:10.1093/hmg/5.12.1851. PMID 8968735.
- Gulati S, Baker P, Li YN, et al. (1997). "Defects in human methionine synthase in cblG patients.". Hum. Mol. Genet. 5 (12): 1859–65. doi:10.1093/hmg/5.12.1859. PMID 8968736.
- Leclerc D, Campeau E, Goyette P, et al. (1997). "Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders.". Hum. Mol. Genet. 5 (12): 1867–74. doi:10.1093/hmg/5.12.1867. PMID 8968737.
- Chen LH, Liu ML, Hwang HY, et al. (1997). "Human methionine synthase. cDNA cloning, gene localization, and expression.". J. Biol. Chem. 272 (6): 3628–34. doi:10.1074/jbc.272.6.3628. PMID 9013615.
- Wilson A, Leclerc D, Saberi F, et al. (1998). "Functionally null mutations in patients with the cblG-variant form of methionine synthase deficiency.". Am. J. Hum. Genet. 63 (2): 409–14. doi:10.1086/301976. PMC 1377317. PMID 9683607. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1377317.
- Salomon O, Rosenberg N, Zivelin A, et al. (2002). "Methionine synthase A2756G and methylenetetrahydrofolate reductase A1298C polymorphisms are not risk factors for idiopathic venous thromboembolism.". Hematol. J. 2 (1): 38–41. doi:10.1038/sj/thj/6200078. PMID 11920232.
- Watkins D, Ru M, Hwang HY, et al. (2002). "Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L.". Am. J. Hum. Genet. 71 (1): 143–53. doi:10.1086/341354. PMC 384971. PMID 12068375. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=384971.
- De Marco P, Calevo MG, Moroni A, et al. (2002). "Study of MTHFR and MS polymorphisms as risk factors for NTD in the Italian population.". J. Hum. Genet. 47 (6): 319–24. doi:10.1007/s100380200043. PMID 12111380.
- Doolin MT, Barbaux S, McDonnell M, et al. (2003). "Maternal genetic effects, exerted by genes involved in homocysteine remethylation, influence the risk of spina bifida.". Am. J. Hum. Genet. 71 (5): 1222–6. doi:10.1086/344209. PMC 385102. PMID 12375236. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=385102.
- Zhu H, Wicker NJ, Shaw GM, et al. (2004). "Homocysteine remethylation enzyme polymorphisms and increased risks for neural tube defects.". Mol. Genet. Metab. 78 (3): 216–21. doi:10.1016/S1096-7192(03)00008-8. PMID 12649067.
External links
- GeneReviews/NCBI/NIH/UW entry on Disorders of Intracellular Cobalamin Metabolism
- ENZYME: EC 2.1.1.13
- MeSH 5-Methyltetrahydrofolate-Homocysteine+S-Methyltransferase
PDB gallery Proteins: enzymes Topics Types EC1 Oxidoreductases/list · EC2 Transferases/list · EC3 Hydrolases/list · EC4 Lyases/list · EC5 Isomerases/list · EC6 Ligases/listB enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 Transferase: one carbon transferases (EC 2.1) 2.1.1: Methyl- N-O-Betaine-homocysteine methyltransferase - Homocysteine methyltransferase - Methionine synthaseOther2.1.2: Hydroxymethyl-,
Formyl- and RelatedHydroxymethyltransferaseFormyltransferaseOther2.1.3: Carboxy- and Carbamoyl CarboxyCarbamoyl2.1.4: Amidino Arginine:glycine amidinotransferaseB enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 Metabolism: amino acid metabolism · synthesis and catabolism enzymes (essential in CAPS) K→acetyl-CoA (see below)G G→pyruvate
→citrateG→glutamate→
α-ketoglutarate→alpha-ketoglutarate→TCAOthergeneration of homocysteine: Methionine adenosyltransferase · Adenosylhomocysteinase
regeneration of methionine: Methionine synthase/Homocysteine methyltransferase · Betaine-homocysteine methyltransferase
conversion to cysteine: Cystathionine beta synthase · Cystathionine gamma-lyase→succinyl-CoA→TCAG→fumarateasparagine→aspartate→This transferase article is a stub. You can help Wikipedia by expanding it.