- Dioxosuccinic acid
-
Dioxosuccinic acid 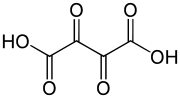

Identifiers CAS number 7580-59-8  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dioxosuccinic acid or dioxobutanedioic acid is an organic compound with formula C4H2O6 or HO-(C=O)4-OH.
Removal of two protons from the molecule would yield the dioxosuccinate anion, C4O62− or (O-(C=O)4-O)2−. This is one of the oxocarbon anions, which consist solely of carbon and oxygen. The name is also used for salts containing that anion, and for esters with the [-O-(C=O)4-O-] moiety.
Removal of a single proton would result in the monovalent anion hydrogendioxosuccinate, C4HO6 − or (HO-(C=O)4-O)−.
Contents
Occurrence
Dioxosuccinic acid is one of the acids occurring naturally in wine, from the oxidation of tartaric acid via dihydroxyfumaric acid.[1]
Reactions
The acid combines with two molecules of water to produce dihydroxytartaric acid, C4H6O8 or HO-(C=O)-(C(OH)2)2-(C=O)-OH. Indeed the product traded under the name "dioxosuccinic acid hydrate" appears to be that substance.
On the other hand, dihydroxytartaric acid behaves like dioxosuccinic acid in some reactions; for example, it reacts with ethanol in the presence of hydrogen chloride to yield the ester diethyl dioxosuccinate.[2]:p.187
See also
- Mesoxalic acid
- Oxaloacetic acid (or oxosuccinic acid)
- Fumaric acid
References
- ^ By Ján Farkaš, Beatrix Farkas (1988), Technology and Biochemistry of Wine.CRC Press, 744 pages. ISBN 2881240704.
- ^ Victorian College of Pharmacy., Dept. of Chemistry (1959), Notes on qualitative analysis.
Categories:- Oxoanions
- Keto acids
Wikimedia Foundation. 2010.
