- Cyclic nucleotide-gated ion channel
-
Cyclic nucleotide-gated (CNG) ion channels are ion channels that function in response to the binding of cyclic nucleotides. CNG channels are nonselective cation channels that are found in the membranes of various types of cells.
Contents
Discovery
The discovery of CGN channels was related to the discovery of intracellular messengers responsible for the mediation of responses in retinal photoreceptors. Before their discovery, it was thought that cyclic nucleotides played a role in phosphorylation. In 1985, it was discovered that cGMP was able to directly activate the light-dependent response of rod channels by studying light-adapted retina of frogs.[1] Soon, CNG channels were found in cone photoreceptors, chemo sensitive cilia of olfactory sensory neurons, and the pineal gland. Cloning and functional expression of CNG channels followed, after the identification of amino acids from purified proteins. Molecular cloning allowed for the discovery of similar channels in many other tissues.[2][3]
Function
CNG channels are directly activated by cyclic nucleotides. Around 4 cyclic nucleotides are needed to activate a channel. CNG channels are nonselective and allow many alkali ions to flow into or out of the cell expressing the channels on it membrane. This results in either depolarization or hyperpolarization. CGN channels can be activated by cAMP or cGMP, or sometimes both. Some channels are more selective while other channels are not. The main role of GNC is sensory transduction in various tissues. Many studies have shown CNG channels in rod and cone receptors. CNG channels also have been found in brain, heart, kidney, and gonads.[3]
Structure
A NCG channel consists of four subunits around a central pore. Each subunit consists 6 transmembrane segments (S1-S6), a P-loop, intracellular amino terminal region, and carboxy terminal region. The P-loop and S6 segments around the pore, which plays a role in ion conduction. There is a cyclic nucleotide binding domain (CNBD) and connection region to the S6 segment in the carboxy terminal. There is a post-CNDB region in the amino terminal.[4]
Alpha subunits
Cyclic nucleotide gated channel alpha subunits include
- Cyclic nucleotide-gated channel alpha 1
- Cyclic nucleotide-gated channel alpha 2
- Cyclic nucleotide-gated channel alpha 3
- Cyclic nucleotide-gated channel alpha 4
Beta subunits
Cyclic nucleotide gated channel beta-subunits include:
- Cyclic nucleotide-gated channel beta 1
- Cyclic nucleotide-gated channel beta 3
Pore
The structure of the pore is similar to other ion channels that contain P-loops. The P-loop enters the membrane of the pore from the extracelluar side and exists to the extracelluar side. The P loop enters as an alpha helix and exists as an uncoiled strand. Helices that cover the inner membrane line the channel. These also form a 6 helix bundle that signifies the entrance. In order to open the pore, a conformation change must occur in the inner 6 helix bundle.[4]
Cyclic nucleotide binding domain
A cyclic nucleotide binding domain is an intracellular domain located in the C-terminal and has a similar sequence to other cyclic nucleotide-binding proteins. The domain is believed to have a ß-pleated sheet and two α-helices. The ß-pleated sheet is antiparallel and 8 stranded. The α helices are named B and C helices. A ligand initially binds to the ß-pleated sheet by following an opening allosteric transition involving the movement to an α-helix toward the ß-pleated sheet. The α-helix is flexible in closed channels. When an α-helix of a CNGA1 subunit is in close proximity to another α-helix, they form intersubunit disulfide bonds. This occurs mainly in closed channels, inhibiting movement of the α-helix towards the ß-pleated sheet. When a ligand binds to the ß-pleated sheet, the now bind ligand stabilizes the movement of the α-helix toward the ß-pleated sheet of each subunit, pulling the α-helices away from each other.[4][5]
C-Linker
The C-Linker is a region that connects the CNBD to the S6 segment. There are many residues that play a role in modulation of CNG channels. This process uses metals such as nickel, zinc, copper, and magnesium. The C-linker region is involved in the coupling of ligand binding to the opening of the pore. The C linker region forms disulfide bonds with N-terminal regions. Disulfide bonds alter the channel function therefore they most likely lie close to the tertiary structure. Disulfide bonds decrease the free energy of the open state compared to the closed state. The specific cysteine residue C481 on the C-linker region is located only a few amino acids away from the binding domain. In the closed state C481 is nonreactive; C481 must undergo a conformational change so that it is accessible for the opening of the channel. Disulfide bonds form between neighboring subunits and C481. Simultaneously there is a C35 cysteine residue at the N-terminal of the C-linker region that can reach two C481 residues, making a favorable disulfide bond compared to a C481-C481 bond.[4] [5]
S6 region
Spontaneous disulfide bond formation is state-dependent, implying that the conformational change in the helix bundle is affiliated with channel gating. When the cyclic nucleotide-gated ion channels are closed, the cytoplasmic ends of the S6 helices are in close proximity to each other. Small cations are able to move through an opening, which implies that the gate is beyond the helix bundle and that S6 helices are in conjunction with conformational changes in the selectivity filter.[5]
P region
The P region forms a loop connecting the S5 and S6 regions which extend to the central axis of the channel. Ionic properties are determined by the residues in the loop between S5 and S6 transmembrane segments. The P region dictates the ion selectivity of the cyclic-nucleotide gated ion channel, which also determine the pore diameter of CNG channels. The P region functions as a channel gate since it prevents ion permeation in the closed state. The pore may be hindered by small conformational changes in this region. The P region acts as a ion selectivity filter that changes structure in the open conformation. The four identical subunits sacrifice a single P region loop from selectivity filter in the open state.[5]
Physiological significance
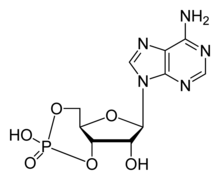
Photoreceptors
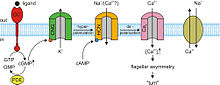
In the absence of light, the cyclic nucleotide guanosine 3’-5’-cyclic monophosphate (cGMP) binds to CNGCs in photoreceptors. This binding causes the channels to open, which allows sodium (Na+) and calcium (Ca2+) ions to flow into the cell causing the outer segment of the photoreceptor to depolarize. This depolarizing flow of ions is known as the dark current. When the retina of the eye detects light, a reaction known as a phototransduction cascade occurs. It is a signal transduction pathway that leads to the activation of the enzyme phosphodiesterase, which hydrolyzes cGMP into 5’-GMP, decreasing the concentration of cGMP. In the absence of cGMP, the CNGCs in the photoreceptors close preventing the flow of the aforementioned dark current. This in turn causes a hyperpolarization of the outer segment of the photoreceptor , preventing the propagation of an action potential and the release of glutamate.[3][4]
Olfactory sensory neurons
Almost all responses to odorants in olfactory sensory neurons (OSNs) are facilitated by CNGCs. When an odorant binds to its specific receptor in the chemosenstive cilia membrane, it activated a G protein. This causes a downstream reaction activating the enzyme adenylyl cyclase. This enzyme is responsible for an increase of cAMP concentration within the OSN. cAMP binds to the CNGCs in the OSN membrane opening them, making the cell highly permeable to Ca2+. Calcium ions flow into the cell causing a depolarization. As in all other cell types, CNGCs in OSNs also allow Na+ to flow into the cell. Additionally, the increased Ca2+ concentration inside the cell activates Ca2+-dependent Cl- channels, which causes intracellular Cl- ions to also flow out of the cell augmenting the depolarization event. In addition to cAMP gated ion channels, a small subset of OSNs also has cGMP-selective CNGCs.[3]
In spermatozoa
Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) mediate several cellular responses for instance swimming behavior, acrosomal exocytosis, and chemoattraction. In the sea urchin species, Strongylocentrotus purpuratus, speract, a short peptide, was studied. Speract activates a receptor-type GC and stimulates a rise of intracellular cGMP concentrations. Speract also increases the concentration of calcium. Although there has yet to be any establishment of a direct causal relationship, the previously mentioned observations suggest that cGMAP activates calcium conductance. CNG channels are prime candidates for the calcium-entry pathway, due to their high calcium permeability. CNG channels have yet to be detected by homology screening. In mammals, testicular CNG channel subunits expressed are A3, B1, and B3. Heterologous expression of the A3 subunit was cloned from testis and produced channels that are cGMP sensitive and selective. It is possible that these channels are involved in a cGMP-stimulates calcium influx into the sperm. However a more extensive characterization of the channel has not been accomplished due to low success rate of detecting channel activity. Since A3 subunit knockout mice are fertile, CNG channels could be involved in some form of motility control and even in chemotactic swimming behavior or in the acrosomal exocytosis. However, a receptor-type GC in mammalian sperm has yet to be identified. Mouse sperm express other channels such as CatSper1. Male sterility can be accomplished by disrupting the CatSper1 gene; additionally, the cAMP-induced calcium influx is abolished in mutant mice. CNG channels and CatSper are unrealted because CatSper lacks a cAMP/cGMP-binding site but does need additional subunits to become functional. It is possible that CNG and CatSper subunits assemble to form calcium-permeable and cyclic nucleotide-sensitive ion channels.[3]
In kidney
cGMP-sensitive channels have been analyzed in renal inner medullary collecting duct cells, which influence the body’s electrolyte and fluid balance. CNG channel activity is controlled by the interaction between cGMP-dependent protein kinase and G1 protein. In the cells from an inner medullary collecting duct, the channel exhibits cation selectivity unit conductance, calcium permeability, and pharmacology very similar to cyclic nucleotide-gated ion channels.[3]
In gonads
There has been identification of cyclic nucleotide-gated ion channel subunits A2, A4, and B1 in a neuronal cell line that secrets gonadotropin-releasing hormone (GnRH). The three subunits make up the CNG channels on chemosensitive cilia of OSNs. In high extracellular calcium, the unit conductance of CNG channels in rods and OSNs are significantly smaller than those measured in the neuronal line. It seems doubtful that CNG channels would create large unit conductance.[3]
CNG channel family
In vertebrates, the CNG channel gene family consists of six members. These genes are divided based on sequence similarity into two subtypes CNGA and CNGB . Additional genes that code for CNG channels have been cloned from Caenorhabditis elegans and Drosophila melanogaster. A subunit of a CNG channel CNGA1, previously called the rod α subunit, was expressed in rod photoreceptors and produced functional channels that were gated by cGMP (cyclic guanosine monophosphate) when expressed externally in either Xenopus oocytes or in a human embryonic kindney cell line (HEK293). In humans, mutated CNGA1 genes result in an autosomal recessive form of retinitis pigmentosa, a degenerative form of blindness. CNGB1, previously called the rod β subunit, is a second subunit of the rod channel. Unlike CNGA1, CNGB1 subunits expressed alone do not produce functional CNG channels, but coexpression of CNGA1 and CNGB1 subunits produces heteromeric channels with modulation, permeation, pharmacology, and cyclic-nucleotide specificity comparable to that of native channels. CNG channels form tetramers, and recent studies indicate that native rod channels consist of three CNGA1 subunits and one CNGB1 subunit. CNGA3 subunits, previously called the cone α subunits, form functional channels when its expression occurs exogenously. On the other hand, CNGB3, previously called the cone β subunit, does not. Mutations in human CNGA3 and CNGB3 are involved in complete achromatopsia, which is a rare, autosomal recessive inherited and congenital disorder characterized by the complete failure in color distinction. CNGA2, previously called the olfactory α subunit and CNGA4, previously called the olfactory β subunit, are involved in transduction of odorant signals in olfactory neurons for which the subunit stoichiometry and arrangement are unknown.[6]
In invertebrates, a CNG channel subunit called CNG-P1 has been cloned from D. melanogaster and is expressed in antennae and the visual system, an indication that CNG channels may be linked to the transduction of light in invertebrates. A second putative CNG-like subunit called CNGL, cloned from D. melanogaster, is found to be expressed in the brain. Two CNG channel subunits, Tax-2 and Tax-4, have been cloned in C. elegans and are responsible for chemosensation, thermosensation, and normal axon outgrowth of some sensory neurons in C. elegans.[6]
Ligand selectivity
By measuring the currents activated in excised inside-out membrane patches upon superfusion with varying ligand concentrations, the ligand sensitivity and selectivity of both native and exogenously expressed CNG channels have been studied. All native CNG channels react to both cAMP and cGMP, but smaller concentrations of cGMP than those of cAMP are needed to activate and open the channels. CNG channels are sharply selective between cGMP and cAMP in rods and cones, whereas in OSNs, the channels respond equally well to both ligands. CNG channels found in OSNs are much more sensitive to both cGMP and cAMP than photoreceptor CNG channels. Studies of dose response relations showed that channel activation is greatly dependent on cGMP concentration; several cGMP molecules bind to the channel in a cooperative manner. Since each subunit contains a single cNMP-binding site, and homo- and heteromeric channels most likely form a tetrameric complex, a maxiumum of four ligand molecules can bind to the channel. Selectivity can be achieved by differential control of the affinity for binding of the ligand, efficacy of gating, or a combination of both. Binding affinity means how tightly cyclic nucleotides bind to the channel. Efficacy refers to the ability of ligand to activate and open the channel once it is bound. Although these processes are useful in understanding selectivity, they are inextricably coupled to each other that it is very difficult to experimentally separate one from another.[3]
Significance in plants
Cyclic nucleotide-gated ions channels in plants are similar in amino acid sequence and structure to non-selective cation CNG channels in animals, as well as trans-membrane-domain K+-selective shaker family channels. However, there are drastic differences that are seen exclusively in plant CNG channels. The amino acid sequence of the pore sequence in plant CNG channels lacks the selectivity filter found in animal CNG channels as well as lacks a GYGD K+-selectivity filter sequence. Other sequence differences are seen in plant CNG channels s, particularly in the cyclic nucleotide binding domain (CNBD). In plants, the CaM binding domain (CaMBD) is found to overlap α-helix C in the CNBD of CNG channels. In animals the CaMBDs are located far away from the CNBD.[7]
CNG channels play a large role in plant immunity and response to pathogens. They have also been implicated in apoptosis in plants. Cyclic nucleotide-gated ion channels are also thought to be involved in pollen development in plants, however its exact role in this mechanism is still not known.[7]
Unlike animal CNG channels, plant CNG channels have not been extensively analyzed biochemically with respect to their structure.[7]
Future research
Researchers have answered many important questions regarding cyclic nucleotide gated ion channels functions in vision and olfaction. In other physiological areas, the role of CNG channels is less defined. With technological growth, there now exists more possibilities for understanding these mechanisms.[3]
Because nitrous oxide (NO) is involved in the stimulation of the synthesis of cGMP, further research is being conducted to understand the physiological interaction of NO with CNG channels, particularly in the covalent modification of CNG channels in OSNs.[3]
References
- ^ Fesenko EE, Kolesnikov SS, Lyubarsky AL (1985). "Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment". Nature 313 (6000): 310–3. doi:10.1038/313310a0. PMID 2578616.
- ^ Yau KW (April 1994). "Cyclic nucleotide-gated channels: an expanding new family of ion channels". Proc. Natl. Acad. Sci. U.S.A. 91 (9): 3481–3. PMC 43603. PMID 7513422. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=43603.
- ^ a b c d e f g h i j Kaupp UB, Seifert R (July 2002). "Cyclic nucleotide-gated ion channels". Physiol. Rev. 82 (3): 769–824. doi:10.1152/physrev.00008.2002. PMID 12087135.
- ^ a b c d e Matulef K, Zagotta WN (2003). "Cyclic nucleotide-gated ion channels". Annu. Rev. Cell Dev. Biol. 19: 23–44. doi:10.1146/annurev.cellbio.19.110701.154854. PMID 14570562.
- ^ a b c d Wang Z, Jiang Y, Lu L, Huang R, Hou Q, Shi F (June 2007). "Molecular mechanisms of cyclic nucleotide-gated ion channel gating". J Genet Genomics 34 (6): 477–85. doi:10.1016/S1673-8527(07)60052-6. PMID 17601606.
- ^ a b Matulef K, Zagotta WN (2003). "Cyclic nucleotide-gated ion channels". Annu. Rev. Cell Dev. Biol. 19: 23–44. doi:10.1146/annurev.cellbio.19.110701.154854. PMID 14570562.
- ^ a b c Kaplan B, Sherman T, Fromm H (May 2007). "Cyclic nucleotide-gated channels in plants". FEBS Lett. 581 (12): 2237–46. doi:10.1016/j.febslet.2007.02.017. PMID 17321525.
Ca2+: Calcium channel Ligand-gatedNa+: Sodium channel Constitutively activeProton gatedK+: Potassium channel Kvα1-6 (1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8) · (2.1, 2.2) · (3.1, 3.2, 3.3, 3.4) · (4.1, 4.2, 4.3) · (5.1) · (6.1, 6.2, 6.3, 6.4)
Kvα7-12 (7.1, 7.2, 7.3, 7.4, 7.5) · (8.1, 8.2) · (9.1, 9.2, 9.3) · (10.1, 10.2) · (11.1/hERG, 11.2, 11.3) · (12.1, 12.2, 12.3)
Kvβ (1, 2, 3) · KCNIP (1, 2, 3, 4) · minK/ISK · minK/ISK-like · MiRP (1, 2, 3) · Shaker geneOther HVCN1GeneralCategories:- Membrane biology
- Ion channels
Wikimedia Foundation. 2010.