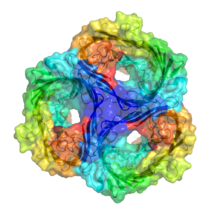
- Porin (protein)
-
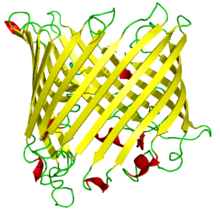 A single monomer of the same protein in side view, illustrating the antiparallel beta barrel structure.
A single monomer of the same protein in side view, illustrating the antiparallel beta barrel structure.
Porins are beta barrel proteins that cross a cellular membrane and act as a pore through which molecules can diffuse.[1] Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules. They are present in the outer membrane of Gram-negative bacteria and some Gram-positive bacteria of the group Mycolata (mycolic acid-containing actinomycetes), the mitochondria, and the chloroplast.
Contents
Structure
Porins are composed of β strands, which are, in general, linked together by beta turns on the cytoplasmic side and long loops of amino acids on the other. The β strands lie in an antiparallel fashion and form a cylindrical tube, called a β barrel.[2] The amino acid composition of the porin β strands are unique in that polar and nonpolar residues alternate along them. This means that the nonpolar residues face outward so as to interact with the nonpolar lipid membrane, whereas the polar residues face inwards into the center of the beta barrel to interact with the aqueous channel. The phospholipids that compose the outer membrane give it the same semi-permeable characteristics as the cytoplasmic membrane.[citation needed]
The porin channel is partially blocked by a loop, called the eyelet, which projects into the cavity. In general, it is found between strands 5 and 6 of each barrel, and it defines the size of solute that can traverse the channel. It is lined almost exclusively with charged amino acids arranged on opposite sides of the channel, creating a transversal electric field across the pore. The eyelet has a local surplus of negative charges from four glutamic acid and seven aspartic acid residues (in contrast to one histidine, two lysine and three arginine residues) is partially compensated for by two bound calcium atoms, and this asymmetric arrangement of molecules is thought to have an influence in the selection of molecules that can pass through the channel.[3]
Cellular roles
To transport medium-sized or charged molecules across, the molecules move through a porin, a water-filled channel or pore.[citation needed]
Porins typically control the diffusion of small metabolites like sugars, ions, and amino acids.
In gram-negative bacteria, the inner membrane is the major permeability barrier, whereas the outer membrane contains porins, which render it largely permeable to molecules less than about 1500 daltons.
The term "nucleoporin" refers to porins facilitating transport through nuclear pores in the nuclear envelope. However, they are often considered distinct from other porins (they are not classified as porins in MeSH.)
Porins are chemically selective – transport only one group of molecules, or may be specific for one molecule[citation needed]. For antibiotics to be effective against a bacterium, it must pass through the outer membrane, using a porin[citation needed]. Bacteria can develop resistance to the antibiotic by mutating the gene that encodes the porin – the antibiotic is then excluded from passing through the outer membrane[citation needed].
Discoverer
The discovery of porins has been attributed to Hiroshi Nikaido, nicknamed "the porinologist."[4]
See also
References
- ^ MeSH Porins
- ^ Schirmer T (1998). "General and specific porins from bacterial outer membranes". J. Struct. Biol. 121 (2): 101–9. doi:10.1006/jsbi.1997.3946. PMID 9615433.
- ^ Branden and Tooze, Introduction to Protein Structure, second edition
- ^ Klebba PE (December 2005). "The porinologist". J. Bacteriol. 187 (24): 8232–6. doi:10.1128/JB.187.24.8232-8236.2005. PMC 1317029. PMID 16321927. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1317029.
External links
- Overview at University of Hamburg
- UMich Orientation of Proteins in Membranes families/superfamily-181 - OmpG porin
- UMich Orientation of Proteins in Membranes families/superfamily-31 - Trimeric porins
- UMich Orientation of Proteins in Membranes families/superfamily-32 - Sugar porins
Ca2+: Calcium channel Ligand-gatedNa+: Sodium channel Constitutively activeProton gatedK+: Potassium channel Kvα1-6 (1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8) · (2.1, 2.2) · (3.1, 3.2, 3.3, 3.4) · (4.1, 4.2, 4.3) · (5.1) · (6.1, 6.2, 6.3, 6.4)
Kvα7-12 (7.1, 7.2, 7.3, 7.4, 7.5) · (8.1, 8.2) · (9.1, 9.2, 9.3) · (10.1, 10.2) · (11.1/hERG, 11.2, 11.3) · (12.1, 12.2, 12.3)
Kvβ (1, 2, 3) · KCNIP (1, 2, 3, 4) · minK/ISK · minK/ISK-like · MiRP (1, 2, 3) · Shaker geneOther HVCN1PorinGeneralCategories:- Outer membrane proteins
Wikimedia Foundation. 2010.