- N-type calcium channel
-
 Illustration of the major elements in a prototypical synapse. Synapses allow nerve cells to communicate with one another through axons and dendrites, converting electrical impulses into chemical signals.
Illustration of the major elements in a prototypical synapse. Synapses allow nerve cells to communicate with one another through axons and dendrites, converting electrical impulses into chemical signals.
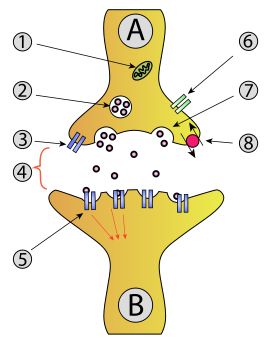 Neuron A (transmitting) to neuron B (receiving)
Neuron A (transmitting) to neuron B (receiving)
- 1. Mitochondrion
- 2. Synaptic vesicle with neurotransmitters
- 3. Autoreceptor
- 4. Synapse with neurotransmitter released (serotonin)
- 5. Postsynaptic receptors activated by neuro-transmitter (induction of a postsynaptic potential)
- 6. Calcium channel
- 7. Exocytosis of a vesicle
- 8. Recaptured neurotransmitter
The N-type calcium channel is a type of voltage-dependent calcium channel. Like the others of this class, the α1 subunit forms the pore through which calcium enters the cell and determines most of the channel's properties. The α1 subunit is also known as the calcium channel, voltage-dependent, N type, alpha 1B subunit (CACNA1B) or Cav2.2[1] which in humans is encoded by the CACNA1B gene.[2][3][4]
Contents
Structure
In addition to the α1 subunit, the following subunits are present in the N-type calcium channel:
Function
N-type ('N' for "Neural-Type" ) calcium channels are found primarily at presynaptic terminals and are involved in neurotransmitter release.[5] Strong depolarization by an action potential causes these channels to open and allow influx of Ca2+, initiating vesicle fusion and release of stored neurotransmitter. N-type channels are blocked by ω-conotoxin.[1]
Therapeutic Potential
Recently, blockade of the N-type calcium channel has emerged as a potential therapeutic strategy for the treatment of alcoholism.[6][7]
Blockers
- ω-Conotoxins
- Ziconotide
- Caroverine
- Cilnidipine
- NP078585[6]
- TROX-1
References
- ^ a b Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Veliçelebi G, Ellis SB, Harpold MM (July 1992). "Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel". Science 257 (5068): 389–95. doi:10.1126/science.1321501. PMID 1321501.
- ^ "Entrez Gene: CACNA1B calcium channel, voltage-dependent, N type, alpha 1B subunit". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=774.
- ^ Diriong S, Lory P, Williams ME, Ellis SB, Harpold MM, Taviaux S (December 1995). "Chromosomal localization of the human genes for alpha 1A, alpha 1B, and alpha 1E voltage-dependent Ca2+ channel subunits". Genomics 30 (3): 605–9. doi:10.1006/geno.1995.1284. PMID 8825650.
- ^ Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (December 2005). "International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels". Pharmacol. Rev. 57 (4): 411–25. doi:10.1124/pr.57.4.5. PMID 16382099.
- ^ Kurihara T, Tanabe T (April 2003). "N-type Ca2+ channel". Nippon Yakurigaku Zasshi. Folia Pharmacologica Japonica 121 (4): 211–22. PMID 12777840. http://joi.jlc.jst.go.jp/JST.JSTAGE/fpj/121.211?lang=en&from=PubMed.
- ^ a b Newton PM, Zeng L, Wang V, Connolly J, Wallace MJ, Kim C, Shin HS, Belardetti F, Snutch TP, Messing RO (November 2008). "A blocker of N- and T-type voltage-gated calcium channels attenuates ethanol-induced intoxication, place preference, self-administration, and reinstatement". J. Neurosci. 28 (45): 11712–9. doi:10.1523/JNEUROSCI.3621-08.2008. PMID 18987207. Lay summary – Pychology Today.
- ^ Newton PM, Messing RO (2009). "The N-type calcium channel is a novel target for treating alcohol use disorders". Channels (Austin) 3 (2): 77–81. PMID 19372737.
Further reading
- Mould J, Yasuda T, Schroeder CI, et al. (2004). "The alpha2delta auxiliary subunit reduces affinity of omega-conotoxins for recombinant N-type (Cav2.2) calcium channels.". J. Biol. Chem. 279 (33): 34705–14. doi:10.1074/jbc.M310848200. PMID 15166237.
- Park SY, Park YT, Kim KE, et al. (2002). "A direct repeat of N-type Ca2+ channel alpha1B gene functions as a negative regulatory element in HeLa cells.". Mol. Cells 13 (2): 341–6. PMID 12018859.
- Calabrese B, Tabarean IV, Juranka P, Morris CE (2002). "Mechanosensitivity of N-type calcium channel currents.". Biophys. J. 83 (5): 2560–74. Bibcode 2002BpJ....83.2560C. doi:10.1016/S0006-3495(02)75267-3. PMC 1302342. PMID 12414690. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1302342.
- Moskvina V, Craddock N, Holmans P, et al. (2009). "Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk.". Mol. Psychiatry 14 (3): 252–60. doi:10.1038/mp.2008.133. PMID 19065143.
- Castiglioni AJ, Raingo J, Lipscombe D (2006). "Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels.". J. Physiol. (Lond.) 576 (Pt 1): 119–34. doi:10.1113/jphysiol.2006.115030. PMC 1995641. PMID 16857708. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1995641.
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (2005). "International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels.". Pharmacol. Rev. 57 (4): 411–25. doi:10.1124/pr.57.4.5. PMID 16382099.
- Yokoyama CT, Myers SJ, Fu J, et al. (2005). "Mechanism of SNARE protein binding and regulation of Cav2 channels by phosphorylation of the synaptic protein interaction site.". Mol. Cell. Neurosci. 28 (1): 1–17. doi:10.1016/j.mcn.2004.08.019. PMID 15607937.
- Maeno-Hikichi Y, Chang S, Matsumura K, et al. (2003). "A PKC epsilon-ENH-channel complex specifically modulates N-type Ca2+ channels.". Nat. Neurosci. 6 (5): 468–75. doi:10.1038/nn1041. PMID 12665800.
- Olsen JV, Blagoev B, Gnad F, et al. (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks.". Cell 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
- Li D, Wang F, Lai M, et al. (2005). "A protein phosphatase 2calpha-Ca2+ channel complex for dephosphorylation of neuronal Ca2+ channels phosphorylated by protein kinase C.". J. Neurosci. 25 (8): 1914–23. doi:10.1523/JNEUROSCI.4790-04.2005. PMID 15728831.
- Butcher AJ, Leroy J, Richards MW, et al. (2006). "The importance of occupancy rather than affinity of CaV(beta) subunits for the calcium channel I-II linker in relation to calcium channel function.". J. Physiol. (Lond.) 574 (Pt 2): 387–98. doi:10.1113/jphysiol.2006.109744. PMC 1817768. PMID 16627564. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1817768.
- Stotz SC, Barr W, McRory JE, et al. (2004). "Several structural domains contribute to the regulation of N-type calcium channel inactivation by the beta 3 subunit.". J. Biol. Chem. 279 (5): 3793–800. doi:10.1074/jbc.M308991200. PMID 14602720.
- Maximov A, Bezprozvanny I (2002). "Synaptic targeting of N-type calcium channels in hippocampal neurons.". J. Neurosci. 22 (16): 6939–52. PMID 12177192.
- Peng S, Hajela RK, Atchison WD (2002). "Characteristics of block by Pb2+ of function of human neuronal L-, N-, and R-type Ca2+ channels transiently expressed in human embryonic kidney 293 cells.". Mol. Pharmacol. 62 (6): 1418–30. doi:10.1124/mol.62.6.1418. PMID 12435810.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2002). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences.". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Murakami M, Fleischmann B, De Felipe C, et al. (2002). "Pain perception in mice lacking the beta3 subunit of voltage-activated calcium channels.". J. Biol. Chem. 277 (43): 40342–51. doi:10.1074/jbc.M203425200. PMID 12161429.
- Vitko I, Shcheglovitov A, Baumgart JP, et al. (2008). "Orientation of the calcium channel beta relative to the alpha(1)2.2 subunit is critical for its regulation of channel activity.". PLoS ONE 3 (10): e3560. doi:10.1371/journal.pone.0003560. PMC 2570331. PMID 18958281. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2570331.
- Coppola T, Magnin-Luthi S, Perret-Menoud V, et al. (2001). "Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin.". J. Biol. Chem. 276 (35): 32756–62. doi:10.1074/jbc.M100929200. PMID 11438518.
- Johnson JM, Castle J, Garrett-Engele P, et al. (2003). "Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays.". Science 302 (5653): 2141–4. doi:10.1126/science.1090100. PMID 14684825.
- Agler HL, Evans J, Tay LH, et al. (2005). "G protein-gated inhibitory module of N-type (ca(v)2.2) ca2+ channels.". Neuron 46 (6): 891–904. doi:10.1016/j.neuron.2005.05.011. PMID 15953418.
External links
Ca2+: Calcium channel Ligand-gatedNa+: Sodium channel Constitutively activeProton gatedK+: Potassium channel Kvα1-6 (1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8) · (2.1, 2.2) · (3.1, 3.2, 3.3, 3.4) · (4.1, 4.2, 4.3) · (5.1) · (6.1, 6.2, 6.3, 6.4)
Kvα7-12 (7.1, 7.2, 7.3, 7.4, 7.5) · (8.1, 8.2) · (9.1, 9.2, 9.3) · (10.1, 10.2) · (11.1/hERG, 11.2, 11.3) · (12.1, 12.2, 12.3)
Kvβ (1, 2, 3) · KCNIP (1, 2, 3, 4) · minK/ISK · minK/ISK-like · MiRP (1, 2, 3) · Shaker geneOther HVCN1GeneralCategories:- Human proteins
- Ion channels
- Electrophysiology
- Membrane biology
- Integral membrane proteins
Wikimedia Foundation. 2010.
