- 5-Methylcytosine
-
5-Methylcytosine 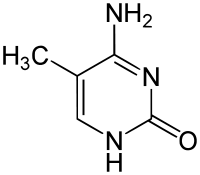 4-amino-5-methyl-3H-pyrimidin-2-one
4-amino-5-methyl-3H-pyrimidin-2-oneIdentifiers CAS number 554-01-8 
PubChem 65040 ChemSpider 58551 
UNII 6R795CQT4H 
KEGG C02376 
MeSH 5-Methylcytosine ChEBI CHEBI:27551 
Jmol-3D images Image 1
Image 2- O=C1/N=C\C(=C(\N)N1)C
Cc1cnc(=O)[nH]c1N
Properties Molecular formula C5H7N3O Molar mass 125.129  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 5-Methylcytosine is a methylated form of the DNA base cytosine that may be involved in the regulation of gene transcription. When cytosine is methylated, the DNA maintains the same sequence, but the expression of methylated genes can be altered (the study of this is called epigenetics).
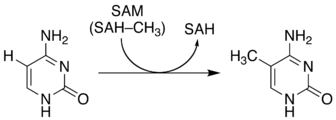
In the figure on the right, a methyl group, is attached to the 5th carbon atom (counting counterclockwise from the NH nitrogen). This methyl group distinguishes 5-methylcytosine from cytosine.
Contents
In vivo
5-Methylcytosine is an epigenetic modification formed by the action of DNA methyltransferases.
The function of this chemical varies significantly among species:[1]
- In bacteria, 5-methylcytosine can be found at a variety of sites, and is often used as a marker to protect DNA from being cut by native methylation-sensitive restriction enzymes.
- In plants, 5-methylcytosine occurs at CpG, CpHpG and CpHpH sequences (where H = A, C or T).
- In fungi and animals, 5-methylcytosine predominantly occurs at CpG dinucleotides. Most eukaryotes methylate only a small percentage of these sites, but 70-80% of CpG cytosines are methylated in vertebrates.
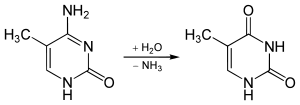
While spontaneous deamination of cytosine forms uracil, which is recognized and removed by DNA repair enzymes, deamination of 5-methylcytosine forms thymine. This conversion of a DNA base from cytosine (C) to thymine (T) can result in a transition mutation. In addition, active enzymatic deamination of cytosine or 5-methylcytosine by the APOBEC family of cytosine deaminases could have beneficial implications on various cellular processes as well as on organismal evolution.[2] The implications of deamination on 5-hydroxymethylcytosine, on the other hand, remains less understood.
In vitro
The NH2 group can be removed (deamination) from 5-Methylcytosine to form thymine with use of reagents such as nitrous acid; cytosine deaminates to uracil under similar conditions.
5-Methylcytosine is resistant to deamination by bisulfite treatment, which deaminates cytosine residues. This property is often exploited to analyze DNA cytosine methylation patterns with bisulfite sequencing.[3]
See also
References
- ^ Colot V, Rossignol JL (1999). "Eukaryotic DNA methylation as an evolutionary device". Bioessays 21 (5): 402–411. doi:10.1002/(SICI)1521-1878(199905)21:5<402::AID-BIES7>3.0.CO;2-B. PMID 10376011.
- ^ Chahwan R., Wontakal S.N., and Roa S. (2010). "Crosstalk between genetic and epigenetic information through cytosine deamination". Trends in Genetics 26 (10): 443–448. doi:10.1016/j.tig.2010.07.005. PMID 20800313.
- ^ Clark SJ, Harrison J, Paul CL, Frommer M (1994). "High sensitivity mapping of methylated cytosines". Nucleic Acids Res. 22 (15): 2990–2997. doi:10.1093/nar/22.15.2990. PMC 310266. PMID 8065911. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=310266.
Literature
- Griffiths, Anthony J. F. (1999). An Introduction to genetic analysis. San Francisco: W.H. Freeman. Chapter 15: Gene Mutation. ISBN 0-7167-3520-2. (available online at the United States National Center for Biotechnology Information)
Categories:- Pyrimidones
- O=C1/N=C\C(=C(\N)N1)C
Wikimedia Foundation. 2010.