- HN3 (nitrogen mustard)
-
Tris(2-chloroethyl)amine 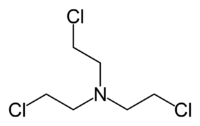
 2-chloro-N,N-bis(2-chloroethyl)ethanamineOther namesHN3, Compound 9560
2-chloro-N,N-bis(2-chloroethyl)ethanamineOther namesHN3, Compound 9560
Nitrogen mustard gas; tris-(2-chloroethyl)amine
trichlormethine; trimustine hydrochlorideIdentifiers CAS number 555-77-1 Jmol-3D images Image 1 - ClCCN(CCCl)CCCl
Properties Molecular formula C6H12Cl3N Molar mass 204.53 g mol−1 Density 1.24 g/cm3 Melting point -3.7 to -4°C
Boiling point 143 °C
 (nitrogen mustard) (verify) (what is:
(nitrogen mustard) (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Tris(2-chloroethyl)amine is the organic compound with the formula N(CH2CH2Cl)3. Often abbreviated HN3, it is a powerful blister agent and a nitrogen mustard gas (although it is not a gas) used for chemical warfare. HN3 was the last of the nitrogen mustard agents developed. It was designed as a military agent and is the only one of the nitrogen mustards that is still used for military purposes. It is the principal representative of the nitrogen mustards because its vesicant properties are almost equal to those of HD and thus the analogy between the two types of mustard is the strongest.[1] As a vesicant the use and production is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.
Contents
Mode of action
Nitrogen mustards react via an initial cyclization to the corresponding iminium salt. The rate of this reaction is pH dependent because the protonated amine cannot cyclize.
Applications
HN-3 has found some applications in chemotherapy, e.g., for Hodgkin's disease, and in some compound Semiconductor research[2] but it is mainly of interest for its military uses and is the only one of these agents that remains anywhere as a military agent. These agents are more immediately toxic than the sulfur mustards.
Exposure
HN-3 can be absorbed into the body by inhalation, ingestion, eye contact, and skin contact (though inhalation is the most common). The chemical is extremely toxic and may damage the eyes, skin, and respiratory tract and suppress the immune system. HN-3 penetrates and binds quickly to cells of the body, but its health effects develop slowly. The full extent of cellular injury may not be known for days.[1]
See also
References
- ^ a b NITROGEN MUSTARD HN-3. Emergency Response Safety and Health Database. National Institute for Occupational Safety and Health. August 22, 2008. Accessed April 10, 2009.
- ^ Benard, C. (1997). Chemical Vapor Deposition. Pennington, NJ, USA: The Electrochemical Society, INC. pp. 78. ISBN 1-56677-178-1.
Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control Categories:- Antineoplastic drugs
- Amines
- Organochlorides
- IARC Group 2B carcinogens
- Nitrogen mustards
Wikimedia Foundation. 2010.
