- Minor spliceosome
-
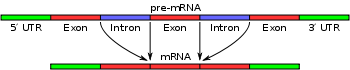
The minor spliceosome is a ribonucleoprotein complex that catalyses the removal (splicing) of an atypical class of spliceosomal introns (U12-type) from eukaryotic messenger RNAs in plant, insects, vertebrates and some fungi (Rhizopus oryzae). This process is called noncanonical splicing, as opposed to U2-dependent canonical splicing. U12-type introns represent less than 1% of all introns in human cells. However they are found in genes performing essential cellular functions.
Contents
Early evidences
A notable feature of eukaryotic nuclear pre mRNA introns is the relatively high level of conservation of the primary sequences of 5’ and 3’ splice sites over a great range of organisms.
Since 1989 till 1991, several groups reported four independent examples of introns with a splice site that differed from the common intron:
- Cartilage matrix protein (CMP/MATN1) gene in human and chicken
- Proliferating cell nucleolar protein P120 (NOL1) gene in human
- mouse Rep3 gene, presumably involved in DNA repair
- Drosophila prospero gene that encodes for a homeobox protein
In 1991 by comparing the intron sequences of P120 and CMP genes, IJ Jackson reported the existence of ATATCC (5') and YYCAC (3') splice sites in these introns. The finding indicated a possible novel splicing mechanism.
In 1994, S.L. Hall and R.A Padgett compared the primary sequence of all reports on the four genes mentioned above. The results suggested a new type of introns with ATATCCTT 5’ splice site and YCCAC 3’ splice site and an almost invariant TCCTTAAC near the 3’ end of the introns (so called 3’ upstream element). A search for small nuclear RNA sequences that are complementary to these splice sites, suggested U12 snRNA (matches 3’ sequence) and U11 snRNA (matches 5’sequence) as being putative factors involved in splicing of this new type of introns.
In all these four genes, the pre-mRNA contains other introns whose sequences conform to those of major class introns. Neither the size nor the position of the AT–AC intron within the host gene is conserved.
In 1996, Woan-Yuh Tarn and Joan A. Steitz described an in vitro system that splices a pre-mRNA substrate containing an AT–AC intron derived from the human P120 gene. Psoralen cross-linking confirms the base-pairing interaction predicted by Hall and Padgett between the branch site of the pre-mRNA substrate and U12 RNA. Native gel electrophoresis reveals that U11, U12, and U5 snRNPs assemble onto the P120 pre-mRNA to form splicing complexes.
Structure of U12-type introns and minor spliceosome
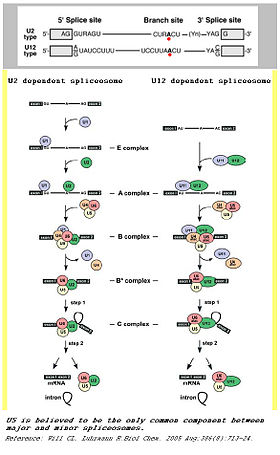
Although originally referred to as ATAC introns, U2-type introns have GT-AG 5’ and 3’ splice sites while U12-type introns have AT-AC at their 5’ and 3’ ends.
The main determinants for distinguishing U2- and U12-type introns are 5’ splice site and branch site sequences.
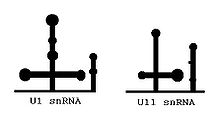
Minor spliceosome consists of U11, U12, U4atac, and U6atac, together with U5 and an unknown number of non-snRNP proteins. The U11, U12 and U4atac/U6atac snRNPs are functional analogs of the U1, U2 and U4/U6 snRNPs in major spliceosome.[1][2][3][4][5] Although the minor U4atac and U6atac snRNAs are functional analogs of U4 and U6, respectively, they share only limited sequence homology (ca. 40%). Furthermore, the sequence of U11 in comparison with U1, as well as U12 compared with U2, are completely unrelated. Despite this fact, the minor U11, U12, U4atac and U6atac snRNAs can be folded into structures similar to U1, U2, U4 and U6, respectively.[6]
Location of minor spliceosomal activity
The location of spliceosomal activity for the minor class spliceosome is regarded by most experts to be in the nucleus. However, a single paper has claimed that the minor spliceosome is active in the cytosol[7]. The data presented within this paper are not fully accepted within the field and directly contradict numerous other papers.
Evolution of minor spliceosome
Like major spliceosome, minor spliceosome had an early origin: several of its characteristic constituents are present in representative organisms from all eukaryotic supergroups for which there is any substantial genome sequence information. In addition, functionally important sequence elements contained within U12-type introns and snRNAs are highly conserved during evolution.
References
Review papers:
- Will CL, Lührmann R (August 2005). "Splicing of a rare class of introns by the U12-dependent spliceosome". Biol. Chem. 386 (8): 713–24. doi:10.1515/BC.2005.084. PMID 16201866. Review.
Classic papers:
- Hall SL, Padgett RA (1994). "Conserved sequences in a class of rare eukaryotic nuclear introns with non-consensus splice sites". J. Mol. Biol. 239 (3): 357–65. doi:10.1006/jmbi.1994.1377. PMID 8201617.
- Jackson IJ (July 25, 1991). "A reappraisal of non-consensus mRNA splice sites". Nucleic Acids Res. 19 (14): 3795–8. doi:10.1093/nar/19.14.3795. PMC 328465. PMID 1713664. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=328465.
- Tarn WY, Steitz JA (March 8, 1996). "A novel spliceosome containing U11, U12, and U5 snRNPs excises a minor class (AT-AC) intron in vitro". Cell 84 (5): 801–11. doi:10.1016/S0092-8674(00)81057-0. PMID 8625417.
- Russell AG, Charette JM, Spencer DF, Gray MW (October 19, 2006). "An early evolutionary origin for the minor spliceosome". Nature 443 (7113): 863–6. doi:10.1038/nature05228. PMID 17051219.
Other references:
- ^ Hall SL, Padgett RA (1996). "Requirement of U12 snRNA for in vivo splicing of a minor class of eukaryotic nuclear pre-mRNA introns". Science 271 (5256): 1716–8. doi:10.1126/science.271.5256.1716. PMID 8596930.
- ^ Tarn WY, Steitz JA (1996). "A novel spliceosome containing U11, U12, and U5 snRNPs excises a minor class (AT-AC) intron in vitro". Cell 84 (5): 801–11. doi:10.1016/S0092-8674(00)81057-0. PMID 8625417.
- ^ Kolossova I, Padgett RA (1997). "U11 snRNA interacts in vivo with the 5' splice site of U12-dependent (AU-AC) pre-mRNA introns". RNA 3 (3): 227–33. PMC 1369475. PMID 9056760. http://www.rnajournal.org/cgi/reprint/3/3/227.
- ^ Yu YT, Steitz JA (1997). "Site-specific crosslinking of mammalian U11 and U6atac to the 5′ splice site of an AT–AC intron". Proc. Natl. Acad. Sci. U.S.A. 94 (12): 6030–5. doi:10.1073/pnas.94.12.6030. PMC 20995. PMID 9177163. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=20995.
- ^ Incorvaia R, Padgett RA (1998). "Base pairing with U6atac snRNA is required for 5' splice site activation of U12-dependent introns in vivo". RNA 4 (6): 709–18. doi:10.1017/S1355838298980207. PMC 1369652. PMID 9622129. http://www.rnajournal.org/cgi/reprint/4/6/709.
- ^ Tarn WY, Steitz JA (1996). "Highly diverged U4 and U6 small nuclear RNAs required for splicing rare AT-AC introns". Science 273 (5283): 1824–32. doi:10.1126/science.273.5283.1824. PMID 8791582.
- ^ König H, Matter N, Bader R, Thiele W, Müller F (Nov 16 2007). "Splicing segregation: the minor spliceosome acts outside the nucleus and controls cell proliferation". Cell. 131 (4): 1718–29. doi:10.1016/j.cell.2007.09.043. PMID 18022366.
See also
Categories:
Wikimedia Foundation. 2010.