- Aryl hydrocarbon receptor
-
"AHR" redirects here. For other uses, see Ahr (disambiguation).
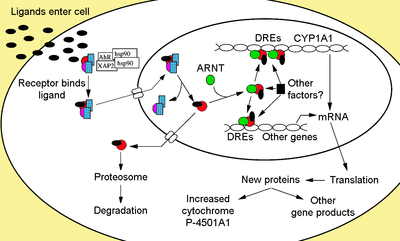
The Aryl hydrocarbon receptor (AhR or AHR) is a member of the family of basic-helix-loop-helix transcription factors. AhR is a cytosolic transcription factor that is normally inactive, bound to several co-chaperones. Upon ligand binding to chemicals such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the chaperones dissociate resulting in AhR translocating into the nucleus and dimerizing with ARNT (AhR nuclear translocator), leading to changes in gene transcription.
Contents
Protein functional domains
The AhR protein contains several domains critical for function and is classified as a member of the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of transcription factors.[1][2] The bHLH motif is located in the N-terminal of the protein and is a common entity in a variety of transcription factors.[3] Members of the bHLH superfamily have two functionally distinctive and highly conserved domains. The first is the basic-region (b), which is involved in the binding of the transcription factor to DNA. The second is the helix-loop-helix (HLH) region, which facilitates protein-protein interactions. Also contained with the AhR are two PAS domains, PAS-A and PAS-B, which are stretches of 200-350 amino acids that exhibit a high sequence homology to the protein domains that were originally found in the Drosophila genes period (Per) and single-minded (Sim) and in AhR’s dimerization partner the aryl hydrocarbon receptor nuclear translocator (ARNT).[4] The PAS domains support specific secondary interactions with other PAS domain containing proteins, as is the case with AhR and ARNT, so that heterozygous and homozygous protein complexes can form. The ligand binding site of AhR is contained within the PAS-B domain[5] and contains several conserved residues critical for ligand binding.[6] Finally, a Q-rich domain is located in the C-terminal region of the protein and is involved in co-activator recruitment and transactivation.[7]
Ligands
Ahr ligands have been generally classified into two categories, synthetic or naturally occurring. The first ligands to be discovered were synthetic and members of the halogenated aromatic hydrocarbons (polychlorinated dibenzodioxins, dibenzofurans and biphenyls) and polycyclic aromatic hydrocarbons (3-methylcholanthrene, benzo(a)pyrene, benzanthracenes and benzoflavones).[8][9] However, recent work has focused on naturally occurring compounds with the hope of identifying an endogenous ligand.
Naturally occurring compounds that have been identified as ligands of Ahr include derivatives of tryptophan such as indigo dye and indirubin,[10] tetrapyroles such as bilirubin,[11] the arachidonic acid metabolites lipoxin A4 and prostaglandin G,[12] modified low-density lipoprotein[13] and several dietary carotinoids.[9] One assumption made in the search for an endogenous ligand is that the ligand will be a receptor agonist. However, work by Savouret et al. has shown this may not be the case since their findings demonstrate that 7-ketocholesterol competitively inhibits Ahr signal transduction.[14]
Signaling pathway
 AhR Signaling Pathway - Denison MS, Nagy SR (2003). "Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals". Annu. Rev. Pharmacol. Toxicol. 43: 309-34. *Reprinted, with permission, from the Annual Review of Pharmacology and Toxicology, Volume 43 (c)2003 by Annual Reviews[9].
AhR Signaling Pathway - Denison MS, Nagy SR (2003). "Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals". Annu. Rev. Pharmacol. Toxicol. 43: 309-34. *Reprinted, with permission, from the Annual Review of Pharmacology and Toxicology, Volume 43 (c)2003 by Annual Reviews[9].
Cytosolic complex
Non-ligand bound Ahr is retained in the cytoplasm as an inactive protein complex consisting of a dimer of Hsp90,[15][16] prostaglandin E synthase 3 (Ptges3, p23)[17][18][19][20] and a single molecule of the immunophilin-like protein hepatitis B virus X-associated protein 2 (XAP2),[21] which was previously identified as AhR interacting protein (AIP)[22] and AhR-activated 9 (ARA9).[23] The dimer of Hsp90, along with p23, has a multifunctional role in the protection of the receptor from proteolysis, constraining the receptor in a conformation receptive to ligand binding and preventing the premature binding of ARNT.[5][18][20][24][25][26] XAP2 interacts with carboxyl-terminal of Hsp90 and binds to the AhR nuclear localization sequence (NLS) preventing the inappropriate trafficking of the receptor into the nucleus.[27][28][29]
Receptor activation
Upon ligand binding to AhR, XAP2 is released resulting in exposure of the NLS, which is located in the bHLH region,[30] leading to importation into the nucleus.[31] It is presumed that once in the nucleus, Hsp90 dissociates exposing the two PAS domains allowing the binding of ARNT.[26][32][33][34] The activated AhR/ARNT heterodimer complex is then capable of either directly and indirectly interacting with DNA by binding to recognition sequences located in the 5’- regulatory region of dioxin-responsive genes.[26][33][35]
DNA binding (xenobiotic response element - XRE)
The classical recognition motif of the AhR/ARNT complex, referred to as either the AhR-, dioxin- or xenobiotic- responsive element (AHRE, DRE or XRE), contains the core sequence 5’-GCGTG-3’[36] within the consensus sequence 5’-T/GNGCGTGA/CG/CA-3’[37][38] in the promoter region of AhR responsive genes. The AhR/ARNT heterodimer directly binds the AHRE/DRE/XRE core sequence in an asymmetric manner such that ARNT binds to 5’-GTG-3’ and AhR binding 5’-TC/TGC-3’.[39][40] Recent research suggests that a second type of element termed AHRE-II, 5’-CATG(N6)C[T/A]TG-3’, is capable of indirectly acting with the AhR/ARNT complex.[41][42] Regardless of the response element, the end result is a variety of differential changes in gene expression.
Functional role in physiology and toxicology
Role in development
In terms of evolution, the oldest physiological role of Ahr is in development. Ahr is presumed to have evolved from invertebrates where it served a ligand-independent role in normal development processes.[43] The Ahr homolog in Drosophila, spineless (ss) is necessary for development of the distal segments of the antenna and leg.[44][45] Ss dimerizes with tango (tgo), which is the homolog to the mammalian Arnt, to initiate gene transcription. Evolution of the receptor in vertebrates resulted in the ability to bind ligand. In developing vertebrates, Ahr seemingly plays a role in cellular proliferation and differentiation.[46] Despite lacking a clear endogenous ligand, AHR appears to play a role in the differentiation of many developmental pathways, including T-cells [47], neurons [48], and hepatocytes [49]. AhR has also been found to have an important function in hematopoietic stem cells: AhR antagonism promotes their self-renewal and ex-vivo expansion [50] and is involved in megakaryocyte differentiation [51].
Adaptive response
The adaptive response is manifested as the induction of xenobiotic metabolizing enzymes. Evidence of this response was first observed from the induction of cytochrome P450, family 1, subfamily A, polypeptide 1 (Cyp1a1) resultant from TCDD exposure, which was determined to be directly related to activation of the Ahr signaling pathway.[52][53][54] The search for other metabolizing genes induced by Ahr ligands, due to the presence of DREs, has led to the identification of an “Ahr gene battery” of Phase I and Phase II metabolizing enzymes consisting of CYP1A1, CYP1A2, CYP1B1, NQO1, ALDH3A1, UGT1A2 and GSTA1.[55] Presumably, vertebrates have this function to be able to detect a wide range of chemicals, indicated by the wide range of substrates Ahr is able to bind and facilitate their biotransformation and elimination.
Toxic response
Extensions of the adaptive response are the toxic responses elicited by Ahr activation. Toxicity results from two different ways of Ahr signaling. The first is a function of the adaptive response in which the induction of metabolizing enzymes results in the production of toxic metabolites. For example, the polycyclic aromatic hydrocarbon benzo(a)pyrene (BaP), a ligand for Ahr, induces its own metabolism and bioactivation to a toxic metabolite via the induction of CYP1A1 and CYP1B1 in several tissues.[56] The second approach to toxicity is the result of aberrant changes in global gene transcription beyond those observed in the “Ahr gene battery.” These global changes in gene expression lead to adverse changes in cellular processes and function. Microarray analysis has proved most beneficial in understanding and characterizing this response.[46][57][58][59]
Protein-protein interactions
In addition to the protein interactions mentioned above, AhR has also been shown to interact with RELA,[60][61] cyclin T1,[62]SRC-1,[63] retinoblastoma protein,[64] NRIP1,[65] estrogen receptor alpha,[66][67] NEDD8[68] and ARNTL.[69]
References
- ^ Burbach KM, Poland A, Bradfield CA (1992). "Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor". Proc. Natl. Acad. Sci. U.S.A. 89 (17): 8185–9. doi:10.1073/pnas.89.17.8185. PMC 49882. PMID 1325649. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=49882.
- ^ Fukunaga BN, Probst MR, Reisz-Porszasz S, Hankinson O (1995). "Identification of functional domains of the aryl hydrocarbon receptor". J. Biol. Chem. 270 (49): 29270–8. doi:10.1074/jbc.270.49.29270. PMID 7493958.
- ^ Jones S (2004). "An overview of the basic helix-loop-helix proteins". Genome Biol. 5 (6): 226. doi:10.1186/gb-2004-5-6-226. PMC 463060. PMID 15186484. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=463060.
- ^ Ema M, Sogawa K, Watanabe N, Chujoh Y, Matsushita N, Gotoh O, Funae Y, Fujii-Kuriyama Y (1992). "cDNA cloning and structure of mouse putative Ah receptor". Biochem. Biophys. Res. Commun. 184 (1): 246–53. doi:10.1016/0006-291X(92)91185-S. PMID 1314586.
- ^ a b Coumailleau P, Poellinger L, Gustafsson JA, Whitelaw ML (1995). "Definition of a minimal domain of the dioxin receptor that is associated with Hsp90 and maintains wild type ligand binding affinity and specificity". J. Biol. Chem. 270 (42): 25291–300. doi:10.1074/jbc.270.42.25291. PMID 7559670.
- ^ Goryo K, Suzuki A, Del Carpio CA, Siizaki K, Kuriyama E, Mikami Y, Kinoshita K, Yasumoto K, Rannug A, Miyamoto A, Fujii-Kuriyama Y, Sogawa K (2007). "Identification of amino acid residues in the Ah receptor involved in ligand binding". Biochem. Biophys. Res. Commun. 354 (2): 396–402. doi:10.1016/j.bbrc.2006.12.227. PMID 17227672.
- ^ Kumar MB, Ramadoss P, Reen RK, Vanden Heuvel JP, Perdew GH (2001). "The Q-rich subdomain of the human Ah receptor transactivation domain is required for dioxin-mediated transcriptional activity". J. Biol. Chem. 276 (45): 42302–10. doi:10.1074/jbc.M104798200. PMID 11551916.
- ^ Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L (2002). "Ligand binding and activation of the Ah receptor". Chem. Biol. Interact. 141 (1–2): 3–24. doi:10.1016/S0009-2797(02)00063-7. PMID 12213382.
- ^ a b c Denison MS, Nagy SR (2003). "Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals". Annu. Rev. Pharmacol. Toxicol. 43: 309–34. doi:10.1146/annurev.pharmtox.43.100901.135828. PMID 12540743.
- ^ Adachi J, Mori Y, Matsui S et al. (2001). "Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine". J. Biol. Chem. 276 (34): 31475–8. doi:10.1074/jbc.C100238200. PMID 11425848.
- ^ Sinal CJ, Bend JR (1997). "Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells". Mol. Pharmacol. 52 (4): 590–9. PMID 9380021.
- ^ Seidel SD, Winters GM, Rogers WJ et al. (2001). "Activation of the Ah receptor signaling pathway by prostaglandins". J. Biochem. Mol. Toxicol. 15 (4): 187–96. doi:10.1002/jbt.16. PMID 11673847.
- ^ McMillan BJ, Bradfield CA (2007). "The aryl hydrocarbon receptor is activated by modified low-density lipoprotein". Proc. Natl. Acad. Sci. U.S.A. 104 (4): 1412–7. doi:10.1073/pnas.0607296104. PMC 1783125. PMID 17227852. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1783125.
- ^ Savouret JF, Antenos M, Quesne M, Xu J, Milgrom E, Casper RF (2001). "7-ketocholesterol is an endogenous modulator for the arylhydrocarbon receptor". J. Biol. Chem. 276 (5): 3054–9. doi:10.1074/jbc.M005988200. PMID 11042205.
- ^ Denis M, Cuthill S, Wikström AC, Poellinger L, Gustafsson JA (1988). "Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor". Biochem. Biophys. Res. Commun. 155 (2): 801–7. doi:10.1016/S0006-291X(88)80566-7. PMID 2844180.
- ^ Perdew GH (1988). "Association of the Ah receptor with the 90-kDa heat shock protein". J. Biol. Chem. 263 (27): 13802–5. PMID 2843537.
- ^ Cox MB, Miller CA (2004). "Cooperation of heat shock protein 90 and p23 in aryl hydrocarbon receptor signaling". Cell Stress Chaperones 9 (1): 4–20. PMC 1065305. PMID 15270073. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1065305.
- ^ a b Kazlauskas A, Poellinger L, Pongratz I (1999). "Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor". J. Biol. Chem. 274 (19): 13519–24. doi:10.1074/jbc.274.19.13519. PMID 10224120.
- ^ Kazlauskas A, Sundström S, Poellinger L, Pongratz I (2001). "The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor". Mol. Cell. Biol. 21 (7): 2594–607. doi:10.1128/MCB.21.7.2594-2607.2001. PMC 86890. PMID 11259606. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=86890.
- ^ a b Shetty PV, Bhagwat BY, Chan WK (2003). "P23 enhances the formation of the aryl hydrocarbon receptor-DNA complex". Biochem. Pharmacol. 65 (6): 941–8. doi:10.1016/S0006-2952(02)01650-7. PMID 12623125.
- ^ Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH (1998). "Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity". Mol. Cell. Biol. 18 (2): 978–88. PMC 108810. PMID 9447995. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=108810.
- ^ Ma Q, Whitlock JP (1997). "A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin". J. Biol. Chem. 272 (14): 8878–84. doi:10.1074/jbc.272.14.8878. PMID 9083006.
- ^ Carver LA, Bradfield CA (1997). "Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo". J. Biol. Chem. 272 (17): 11452–6. doi:10.1074/jbc.272.17.11452. PMID 9111057.
- ^ Carver LA, Jackiw V, Bradfield CA (1994). "The 90-kDa heat shock protein is essential for Ah receptor signaling in a yeast expression system". J. Biol. Chem. 269 (48): 30109–12. PMID 7982913.
- ^ Pongratz I, Mason GG, Poellinger L (1992). "Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors that require hsp90 both for ligand-binding activity and repression of intrinsic DNA binding activity". J. Biol. Chem. 267 (19): 13728–34. PMID 1320028.
- ^ a b c Whitelaw M, Pongratz I, Wilhelmsson A, Gustafsson JA, Poellinger L (1993). "Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor". Mol. Cell. Biol. 13 (4): 2504–14. PMC 359572. PMID 8384309. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=359572.
- ^ Carver LA, LaPres JJ, Jain S, Dunham EE, Bradfield CA (1998). "Characterization of the Ah receptor-associated protein, ARA9". J. Biol. Chem. 273 (50): 33580–7. doi:10.1074/jbc.273.50.33580. PMID 9837941.
- ^ Petrulis JR, Hord NG, Perdew GH (2000). "Subcellular localization of the aryl hydrocarbon receptor is modulated by the immunophilin homolog hepatitis B virus X-associated protein 2". J. Biol. Chem. 275 (48): 37448–53. doi:10.1074/jbc.M006873200. PMID 10986286.
- ^ Petrulis JR, Kusnadi A, Ramadoss P, Hollingshead B, Perdew GH (2003). "The hsp90 Co-chaperone XAP2 alters importin beta recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity". J. Biol. Chem. 278 (4): 2677–85. doi:10.1074/jbc.M209331200. PMID 12431985.
- ^ Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K (1998). "Nuclear localization and export signals of the human aryl hydrocarbon receptor". J. Biol. Chem. 273 (5): 2895–904. doi:10.1074/jbc.273.5.2895. PMID 9446600.
- ^ Pollenz RS, Barbour ER (2000). "Analysis of the complex relationship between nuclear export and aryl hydrocarbon receptor-mediated gene regulation". Mol. Cell. Biol. 20 (16): 6095–104. doi:10.1128/MCB.20.16.6095-6104.2000. PMC 86085. PMID 10913191. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=86085.
- ^ Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, Hankinson O (1991). "Cloning of a factor required for activity of the Ah (dioxin) receptor". Science 252 (5008): 954–8. doi:10.1126/science.1852076. PMID 1852076.
- ^ a b Probst MR, Reisz-Porszasz S, Agbunag RV, Ong MS, Hankinson O (1993). "Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action". Mol. Pharmacol. 44 (3): 511–8. PMID 8396713.
- ^ Reyes H, Reisz-Porszasz S, Hankinson O (1992). "Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor". Science 256 (5060): 1193–5. doi:10.1126/science.256.5060.1193. PMID 1317062.
- ^ Dolwick KM, Swanson HI, Bradfield CA (1993). "In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition". Proc. Natl. Acad. Sci. U.S.A. 90 (18): 8566–70. doi:10.1073/pnas.90.18.8566. PMC 47398. PMID 8397410. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=47398.
- ^ Shen ES, Whitlock JP (1992). "Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor". J. Biol. Chem. 267 (10): 6815–9. PMID 1313023.
- ^ Lusska A, Shen E, Whitlock JP (1993). "Protein-DNA interactions at a dioxin-responsive enhancer. Analysis of six bona fide DNA-binding sites for the liganded Ah receptor". J. Biol. Chem. 268 (9): 6575–80. PMID 8384216.
- ^ Yao EF, Denison MS (1992). "DNA sequence determinants for binding of transformed Ah receptor to a dioxin-responsive enhancer". Biochemistry 31 (21): 5060–7. doi:10.1021/bi00136a019. PMID 1318077.
- ^ Bacsi SG, Reisz-Porszasz S, Hankinson O (1995). "Orientation of the heterodimeric aryl hydrocarbon (dioxin) receptor complex on its asymmetric DNA recognition sequence". Mol. Pharmacol. 47 (3): 432–8. PMID 7700240.
- ^ Swanson HI, Chan WK, Bradfield CA (1995). "DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins". J. Biol. Chem. 270 (44): 26292–302. doi:10.1074/jbc.270.44.26292. PMID 7592839.
- ^ Boutros PC, Moffat ID, Franc MA, Tijet N, Tuomisto J, Pohjanvirta R, Okey AB (2004). "Dioxin-responsive AHRE-II gene battery: identification by phylogenetic footprinting". Biochem. Biophys. Res. Commun. 321 (3): 707–15. doi:10.1016/j.bbrc.2004.06.177. PMID 15358164.
- ^ Sogawa K, Numayama-Tsuruta K, Takahashi T, Matsushita N, Miura C, Nikawa J, Gotoh O, Kikuchi Y, Fujii-Kuriyama Y (2004). "A novel induction mechanism of the rat CYP1A2 gene mediated by Ah receptor-Arnt heterodimer". Biochem. Biophys. Res. Commun. 318 (3): 746–55. doi:10.1016/j.bbrc.2004.04.090. PMID 15144902.
- ^ Hahn ME, Karchner SI, Evans BR, Franks DG, Merson RR, Lapseritis JM (2006). "Unexpected diversity of aryl hydrocarbon receptors in non-mammalian vertebrates: insights from comparative genomics". J. Exp. Zoolog. Part a Comp. Exp. Biol. 305 (9): 693–706. doi:10.1002/jez.a.323. PMID 16902966.
- ^ Duncan DM, Burgess EA, Duncan I (1998). "Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor". Genes Dev. 12 (9): 1290–303. doi:10.1101/gad.12.9.1290. PMC 316766. PMID 9573046. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=316766.
- ^ Emmons RB, Duncan D, Estes PA et al. (1999). "The spineless-aristapedia and tango bHLH-PAS proteins interact to control antennal and tarsal development in Drosophila". Development 126 (17): 3937–45. PMID 10433921.
- ^ a b Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R (2006). "Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries". Mol. Pharmacol. 69 (1): 140–53. doi:10.1124/mol.105.018705. PMID 16214954.
- ^ Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL (2008). "Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor". Nature 1 (453): 65–71. doi:10.1038/nature06880. PMID 18362915.
- ^ Akahoshi E, Yoshimura S, Ishihara-Sugano M. (2006). "Over-expression of AhR (aryl hydrocarbon receptor) induces neural differentiation of Neuro2a cells: neurotoxicology study". Environ Health. 1 (5): 24. doi:10.1186/1476-069X-5-24. PMC 1570454. PMID 16956419. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1570454.
- ^ Walisser JA, Glover E, Pande K, Liss AL, Bradfield CA. (2005). "Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types". Proc Natl Acad Sci U S A 102 (49): 17858–63. doi:10.1073/pnas.0504757102. ISBN 0504757102. PMC 1308889. PMID 16301529. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1308889.
- ^ http://www.sciencemag.org/content/329/5997/1345.abstract
- ^ Lindsey S, Papoutsakis ET (2011). "The aryl hydrocarbon receptor (AHR) transcription factor regulates megakaryocytic polyploidization". Br J Haematol. 152 (4): 469–84. doi:10.1111/j.1365-2141.2010.08548.x. PMID 21226706.
- ^ Israel DI, Whitlock JP (1983). "Induction of mRNA specific for cytochrome P1-450 in wild type and variant mouse hepatoma cells". J. Biol. Chem. 258 (17): 10390–4. PMID 6885786.
- ^ Israel DI, Whitlock JP (1984). "Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells". J. Biol. Chem. 259 (9): 5400–2. PMID 6715350.
- ^ Ko HP, Okino ST, Ma Q, Whitlock JP (1996). "Dioxin-induced CYP1A1 transcription in vivo: the aromatic hydrocarbon receptor mediates transactivation, enhancer-promoter communication, and changes in chromatin structure". Mol. Cell. Biol. 16 (1): 430–6. PMC 231019. PMID 8524325. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=231019.
- ^ Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP (2000). "Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis". Biochem. Pharmacol. 59 (1): 65–85. doi:10.1016/S0006-2952(99)00310-X. PMID 10605936.
- ^ Harrigan JA, Vezina CM, McGarrigle BP et al. (2004). "DNA adduct formation in precision-cut rat liver and lung slices exposed to benzo[a]pyrene". Toxicol. Sci. 77 (2): 307–14. doi:10.1093/toxsci/kfh030. PMID 14691214.
- ^ Martinez JM, Afshari CA, Bushel PR, Masuda A, Takahashi T, Walker NJ (2002). "Differential toxicogenomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin in malignant and nonmalignant human airway epithelial cells". Toxicol. Sci. 69 (2): 409–23. doi:10.1093/toxsci/69.2.409. PMID 12377990.
- ^ Vezina CM, Walker NJ, Olson JR (2004). "Subchronic exposure to TCDD, PeCDF, PCB126, and PCB153: effect on hepatic gene expression". Environ. Health Perspect. 112 (16): 1636–44. doi:10.1289/ehp.7253. PMC 1247661. PMID 15598615. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1247661.
- ^ Ovando BJ, Vezina CM, McGarrigle BP, Olson JR (2006). "Hepatic gene downregulation following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin". Toxicol. Sci. 94 (2): 428–38. doi:10.1093/toxsci/kfl111. PMID 16984957.
- ^ Kim, D W; Gazourian L, Quadri S A, Romieu-Mourez R, Sherr D H, Sonenshein G E (Nov. 2000). "The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells". Oncogene (England) 19 (48): 5498–506. doi:10.1038/sj.onc.1203945. ISSN 0950-9232. PMID 11114727.
- ^ Ruby, Carl E; Leid Mark, Kerkvliet Nancy I (Sep. 2002). "2,3,7,8-Tetrachlorodibenzo-p-dioxin suppresses tumor necrosis factor-alpha and anti-CD40-induced activation of NF-kappaB/Rel in dendritic cells: p50 homodimer activation is not affected". Mol. Pharmacol. (United States) 62 (3): 722–8. doi:10.1124/mol.62.3.722. ISSN 0026-895X. PMID 12181450.
- ^ Tian, Yanan; Ke Sui, Chen Min, Sheng Tao (Nov. 2003). "Interactions between the aryl hydrocarbon receptor and P-TEFb. Sequential recruitment of transcription factors and differential phosphorylation of C-terminal domain of RNA polymerase II at cyp1a1 promoter". J. Biol. Chem. (United States) 278 (45): 44041–8. doi:10.1074/jbc.M306443200. ISSN 0021-9258. PMID 12917420.
- ^ Beischlag, Timothy V; Wang Song, Rose David W, Torchia Joseph, Reisz-Porszasz Suzanne, Muhammad Khurshid, Nelson Walter E, Probst Markus R, Rosenfeld Michael G, Hankinson Oliver (Jun. 2002). "Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex". Mol. Cell. Biol. (United States) 22 (12): 4319–33. doi:10.1128/MCB.22.12.4319-4333.2002. ISSN 0270-7306. PMC 133867. PMID 12024042. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=133867.
- ^ Ge, N L; Elferink C J (Aug. 1998). "A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle". J. Biol. Chem. (UNITED STATES) 273 (35): 22708–13. doi:10.1074/jbc.273.35.22708. ISSN 0021-9258. PMID 9712901.
- ^ Kumar, M B; Tarpey R W, Perdew G H (Aug. 1999). "Differential recruitment of coactivator RIP140 by Ah and estrogen receptors. Absence of a role for LXXLL motifs". J. Biol. Chem. (UNITED STATES) 274 (32): 22155–64. doi:10.1074/jbc.274.32.22155. ISSN 0021-9258. PMID 10428779.
- ^ Wormke, Mark; Stoner Matthew, Saville Bradley, Walker Kelcey, Abdelrahim Maen, Burghardt Robert, Safe Stephen (Mar. 2003). "The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes". Mol. Cell. Biol. (United States) 23 (6): 1843–55. doi:10.1128/MCB.23.6.1843-1855.2003. ISSN 0270-7306. PMC 149455. PMID 12612060. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=149455.
- ^ Klinge, C M; Kaur K, Swanson H I (Jan. 2000). "The aryl hydrocarbon receptor interacts with estrogen receptor alpha and orphan receptors COUP-TFI and ERRalpha1". Arch. Biochem. Biophys. (UNITED STATES) 373 (1): 163–74. doi:10.1006/abbi.1999.1552. ISSN 0003-9861. PMID 10620335.
- ^ Antenos, Monica; Casper Robert F, Brown Theodore J (Nov. 2002). "Interaction with Nedd8, a ubiquitin-like protein, enhances the transcriptional activity of the aryl hydrocarbon receptor". J. Biol. Chem. (United States) 277 (46): 44028–34. doi:10.1074/jbc.M202413200. ISSN 0021-9258. PMID 12215427.
- ^ Hogenesch, J B; Chan W K, Jackiw V H, Brown R C, Gu Y Z, Pray-Grant M, Perdew G H, Bradfield C A (Mar. 1997). "Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway". J. Biol. Chem. (UNITED STATES) 272 (13): 8581–93. doi:10.1074/jbc.272.13.8581. ISSN 0021-9258. PMID 9079689.
External links
Transcription factors and intracellular receptors (1) Basic domains (1.1) Basic leucine zipper (bZIP)Activating transcription factor (AATF, 1, 2, 3, 4, 5, 6, 7) · AP-1 (c-Fos, FOSB, FOSL1, FOSL2, JDP2, c-Jun, JUNB, JUND) · BACH (1, 2) · BATF · BLZF1 · C/EBP (α, β, γ, δ, ε, ζ) · CREB (1, 3, L1) · CREM · DBP · DDIT3 · GABPA · HLF · MAF (B, F, G, K) · NFE (2, L1, L2, L3) · NFIL3 · NRL · NRF (1, 2, 3) · XBP1(1.2) Basic helix-loop-helix (bHLH)ATOH1 · AhR · AHRR · ARNT · ASCL1 · BHLHB2 · BMAL (ARNTL, ARNTL2) · CLOCK · EPAS1 · FIGLA · HAND (1, 2) · HES (5, 6) · HEY (1, 2, L) · HES1 · HIF (1A, 3A) · ID (1, 2, 3, 4) · LYL1 · MESP2 · MXD4 · MYCL1 · MYCN · Myogenic regulatory factors (MyoD, Myogenin, MYF5, MYF6) · Neurogenins (1, 2, 3) · NeuroD (1, 2) · NPAS (1, 2, 3) · OLIG (1, 2) · Pho4 · Scleraxis · SIM (1, 2) · TAL (1, 2) · Twist · USF1(1.3) bHLH-ZIP(1.4) NF-1(1.5) RF-X(1.6) Basic helix-span-helix (bHSH)(2) Zinc finger DNA-binding domains (2.1) Nuclear receptor (Cys4)subfamily 1 (Thyroid hormone (α, β), CAR, FXR, LXR (α, β), PPAR (α, β/δ, γ), PXR, RAR (α, β, γ), ROR (α, β, γ), Rev-ErbA (α, β), VDR)
subfamily 2 (COUP-TF (I, II), Ear-2, HNF4 (α, γ), PNR, RXR (α, β, γ), Testicular receptor (2, 4), TLX)
subfamily 3 (Steroid hormone (Androgen, Estrogen (α, β), Glucocorticoid, Mineralocorticoid, Progesterone), Estrogen related (α, β, γ))
subfamily 4 NUR (NGFIB, NOR1, NURR1) · subfamily 5 (LRH-1, SF1) · subfamily 6 (GCNF) · subfamily 0 (DAX1, SHP)(2.2) Other Cys4(2.3) Cys2His2General transcription factors (TFIIA, TFIIB, TFIID, TFIIE (1, 2), TFIIF (1, 2), TFIIH (1, 2, 4, 2I, 3A, 3C1, 3C2))
ATBF1 · BCL (6, 11A, 11B) · CTCF · E4F1 · EGR (1, 2, 3, 4) · ERV3 · GFI1 · GLI-Krüppel family (1, 2, 3, REST, S2, YY1) · HIC (1, 2) · HIVEP (1, 2, 3) · IKZF (1, 2, 3) · ILF (2, 3) · KLF (2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17) · MTF1 · MYT1 · OSR1 · PRDM9 · SALL (1, 2, 3, 4) · SP (1, 2, 4, 7, 8) · TSHZ3 · WT1 · Zbtb7 (7A, 7B) · ZBTB (16, 17, 20, 32, 33, 40) · zinc finger (3, 7, 9, 10, 19, 22, 24, 33B, 34, 35, 41, 43, 44, 51, 74, 143, 146, 148, 165, 202, 217, 219, 238, 239, 259, 267, 268, 281, 295, 300, 318, 330, 346, 350, 365, 366, 384, 423, 451, 452, 471, 593, 638, 644, 649, 655)(2.4) Cys6(2.5) Alternating composition(3) Helix-turn-helix domains (3.1) HomeodomainARX · CDX (1, 2) · CRX · CUTL1 · DBX (1, 2) · DLX (3, 4, 5) · EMX2 · EN (1, 2) · FHL (1, 2, 3) · HESX1 · HHEX · HLX · Homeobox (A1, A2, A3, A4, A5, A7, A9, A10, A11, A13, B1, B2, B3, B4, B5, B6, B7, B8, B9, B13, C4, C5, C6, C8, C9, C10, C11, C12, C13, D1, D3, D4, D8, D9, D10, D11, D12, D13) · HOPX · IRX (1, 2, 3, 4, 5, 6, MKX) · LMX (1A, 1B) · MEIS (1, 2) · MEOX2 · MNX1 · MSX (1, 2) · NANOG · NKX (2-1, 2-2, 2-3, 2-5, 3-1, 3-2, 6-1, 6-2) · NOBOX · PBX (1, 2, 3) · PHF (1, 3, 6, 8, 10, 16, 17, 20, 21A) · PHOX (2A, 2B) · PITX (1, 2, 3) · POU domain (PIT-1, BRN-3: A, B, C, Octamer transcription factor: 1, 2, 3/4, 6, 7, 11) · OTX (1, 2) · PDX1 · SATB2 · SHOX2 · VAX1 · ZEB (1, 2)(3.2) Paired box(3.3) Fork head / winged helix(3.4) Heat Shock Factors(3.5) Tryptophan clusters(3.6) TEA domain(4) β-Scaffold factors with minor groove contacts (4.1) Rel homology region(4.2) STAT(4.3) p53(4.4) MADS box(4.6) TATA binding proteins(4.7) High-mobility group(4.10) Cold-shock domainCSDA, YBX1(4.11) Runt(0) Other transcription factors (0.2) HMGI(Y)(0.3) Pocket domain(0.6) MiscellaneousCategories:- Human proteins
- Gene expression
- Receptors
- Transcription factors
Wikimedia Foundation. 2010.

