- Polychlorinated biphenyl
-
"PCBs" redirects here. For printed circuit boards, see printed circuit board.
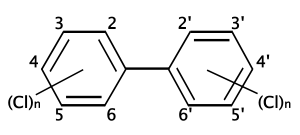
Polychlorinated biphenyls (PCBs; CAS number 1336-36-3 ) are a class of organic compounds (specifically organochlorides) with 2 to 10 chlorine atoms attached to biphenyl, which is a molecule composed of two benzene rings. The chemical formula for PCBs is C12H10-xClx.
PCBs were widely used as dielectric and coolant fluids, for example in transformers, capacitors, and electric motors. Due to PCBs' toxicity and classification as a persistent organic pollutant, PCB production was banned by the United States Congress in 1979 and by the Stockholm Convention on Persistent Organic Pollutants in 2001.[1] Concerns about the toxicity of PCBs are largely based on compounds within this group that share a structural similarity and toxic mode of action with dioxin. Toxic effects such as endocrine disruption and neurotoxicity are also associated with other compounds within the group.
Contents
Physical and chemical properties
PCB congeners are odorless, tasteless, clear to pale-yellow, viscous liquids (highly chlorinated mixtures are more viscous and deeper yellow). They are formed by electrophilic chlorination of biphenyl with chlorine gas. Theoretically, 209 different PCB congeners are possible, although only about 130 are found in commercial PCB mixtures.[1] PCBs have low water solubilities — 0.0027-0.42 ng/L for Aroclors,[2] and low vapor pressures at room temperature, but they have high solubilities in most organic solvents, oils, and fats. They have high dielectric constants, very high thermal conductivity,[2] high flash points (from 170 to 380 °C)[2] and are chemically fairly inert, being extremely resistant to oxidation, reduction, addition, elimination, and electrophilic substitution.[3] The density varies from 1.182 to 1.566 kg/L.[2] Other physical and chemical properties vary widely across the class. As the degree of chlorination increases, melting point and lipophilicity increase, and vapour pressure and water solubility decrease.[2]
PCBs readily penetrate skin, PVC (polyvinyl chloride), and latex (natural rubber).[4] PCB-resistant materials include Viton, polyethylene, polyvinyl acetate (PVA), polytetrafluoroethylene (PTFE), butyl rubber, nitrile rubber, and Neoprene.[4]
In terms of structural relationship to toxicity, PCBs fall into 2 distinct categories, referred to as coplanar or non-ortho-substituted arene substitution patterns and noncoplanar or ortho-substituted congeners. The coplanar group members have a fairly rigid structure, with the 2 phenyl rings in the same plane. This gives the molecule a structure similar to polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans, and allows it to act in the same way as these molecules as an agonist of the aryl hydrocarbon receptor (AhR) in organisms. These type of PCBs are considered as contributors to overall dioxin toxicity, and the term dioxin is often used interchangeably when the environmental and toxic impact of these compounds is considered. Noncoplanar PCBs, with chlorine atoms at the ortho positions, have not been found to activate the AhR, and are not considered part of the dioxin group; however, studies have indicated some neurotoxic and immunotoxic effects, but at levels much higher than normally associated with dioxins, and thus of much less concern to regulatory bodies.[5]
PCBs are very stable compounds and do not decompose readily. Their destruction by chemical, thermal, and biochemical processes is extremely difficult, and presents the risk of generating extremely toxic dibenzodioxins and dibenzofurans through partial oxidation. Intentional degradation as a treatment of unwanted PCBs generally requires high heat or catalysis (see Methods of destruction below).
Alternative names
Commercial PCB mixtures were marketed under the following names.:[2][6]
Brazil
- Ascarel
Former Czechoslovakia
- Delor
France
- Phenoclor
- Pyralène (both used by Prodolec)
Germany
- Clophen (used by Bayer)
Italy
- Apirolio
- Fenclor
Japan
- Kanechlor (used by Kanegafuchi)
- Santotherm (used by Mitsubishi)
- Pyroclor
Former USSR
- Sovol
- Sovtol
United Kingdom
- Aroclor xxxx (used by Monsanto Company)
- Askarel
United States
- Aroclor xxxx (used by Monsanto Company)
- Asbestol
- Askarel
- Bakola131
- Chlorextol - Allis-Chalmers trade name
- Hydol
- Inerteen (used by Westinghouse)
- Noflamol
- Pyranol/Pyrenol (used by General Electric)
- Saf-T-Kuhl
- Therminol
Applications
PCBs were used as coolants and insulating fluids (transformer oil) for transformers and capacitors, such as those used in old fluorescent light ballasts. [7]. PCBs were also used as plasticizers in paints and cements, stabilizing additives in flexible PVC coatings of electrical wiring and electronic components, pesticide extenders, cutting oils, reactive flame retardants, lubricating oils, hydraulic fluids, and sealants (for caulking in schools and commercial buildings[8]), adhesives, wood floor finishes (such as Fabulon and other products of Halowax in the U.S.),[8] paints, de-dusting agents, water-proofing compounds, casting agents, vacuum pump fluids, fixatives in microscopy, surgical implants, and in carbonless copy ("NCR") paper.[2]
History
PCBs, originally termed "chlorinated diphenyls," were commercially produced as complex mixtures containing multiple isomers at different degrees of chlorination. In the United States, commercial production of PCBs was taken over in 1929 by Monsanto Company from Swann Chemical Company. Manufacturing levels increased in response to the electrical industry's need for a "safer" (than flammable mineral oil) cooling and insulating fluid for industrial transformers and capacitors. PCBs were also commonly used as stabilizing additives in the manufacture of flexible PVC coatings for electrical wiring and electronic components to enhance the heat and fire resistance of the PVC.[9]
The toxicity associated with PCBs and other chlorinated hydrocarbons, including polychlorinated naphthalenes, was recognized very early due to a variety of industrial incidents.[10] A conference about the hazards was organized at Harvard School of Public Health in 1937, and a number of publications referring to the toxicity of various chlorinated hydrocarbons were published before 1940.[11] Robert Brown reminded chemists in 1947 that Arochlors were "objectionably toxic. Thus the maximum permissible concentration for an 8-hr. day is 1 mg/m3 of air. They also produce a serious and disfiguring dermatitis".[12] However, PCB manufacture and use continued with few restraints until the 1970s.
PCBs are persistent organic pollutants and have entered the environment through both use and disposal. The environmental transport of PCBs is complex and nearly global in scale. The public, legal, and scientific concerns about PCBs arose from research indicating they are likely carcinogens having the potential to adversely impact the environment and, therefore, undesirable as commercial products. Despite active research spanning five decades, extensive regulatory actions, and an effective ban on their production since the 1970s, PCBs still persist in the environment and remain a focus of attention.[2]
The only North American producer, Monsanto Company, marketed PCBs under the trade name Aroclor from 1930 to 1977. These were sold under trade names followed by a 4-digit number. In general, the first two digits refer to the number of carbon atoms in the biphenyl skeleton (for PCBs this is 12); the second two numbers indicate the percentage of chlorine by mass in the mixture. Thus, Aroclor 1260 has 12 carbon atoms and contains 60% chlorine by mass. An exception is Aroclor 1016, which also has 12 carbon atoms, but has 42% chlorine by mass. Different Aroclors were used at different times and for different applications. In electrical equipment manufacturing in the USA, Aroclor 1260 and Aroclor 1254 were the main mixtures used before 1950; Aroclor 1242 was the main mixture used in the 1950s and 1960s until it was phased out in 1971 and replaced by Aroclor 1016.[2]
Manufacture peaked in the 1960s, by which time the electrical industry had lobbied the U.S. Congress to make them mandatory safety equipment. In 1966, they were determined by Swedish chemist Dr. Soren Jensen to be an environmental contaminant,[13] and it was Dr. Jensen, according to a 1994 article in Sierra, who named them PCBs. Previously, they had simply been called "phenols" or referred to by various trade names, such as Aroclor, Kennechlor, Pyrenol, Chlorinol and others.
Their commercial utility was based largely on their chemical stability, including low flammability, and desirable physical properties, including electrical insulating properties. Their chemical and physical stability has also been responsible for their continuing persistence in the environment, and the lingering interest decades after regulations were imposed to control environmental contamination.
In 1972, PCB production plants existed in Austria, the then Federal Republic of Germany, France, Great Britain, Italy, Japan, Spain, USSR, and USA.[2]
In 1973, the use of PCBs was banned in "open" or "dissipative" sources, such as:
- plasticisers in paints and cements
- casting agents
- fire retardant fabric treatments and heat stabilizing additives for PVC electrical insulation
- adhesives
- paints and water-proofing
- railway sleepers
However, they continued to be allowed in "totally enclosed uses" such as transformers and capacitors, which, in certain failure modes or out-of-specification conditions, can leak, catch fire, or explode. It was Ward B. Stone of the New York State Department of Environmental Conservation (NYSDEC) who first published his findings in the early 1970s that PCBs were leaking from transformers and had contaminated the soil at the bottom of utility poles.[citation needed] Concern over the toxicity and persistence (chemical stability) of PCBs in the environment led the United States Congress to ban their domestic production in 1979,[14] although some use continues in closed systems such as capacitors and transformers.
"Enclosed uses" of PCBs include:
- capacitors
- insulating fluids in transformers
- vacuum pump fluids
- hydraulic fluids
In the UK, closed uses of PCBs in new equipment were banned in 1981, when nearly all UK PCB synthesis ceased, but closed uses in existing equipment containing in excess of 5 litres of PCBs were not stopped until December 2000.[15]
In Japan, PCBs were first produced by Kanegafuchi Chemical Co. Ltd. (Kaneka) in 1954 and production continued until 1972 when the Japanese government banned the production, use, and import of PCBs.[2]
Estimates have put the total global production of PCBs on the order of 1.5 million tons. The United States was the single largest producer with over 600,000 tons produced between 1930 and 1977. The European region follows with nearly 450,000 tons through 1984. It is unlikely that a full inventory of global PCB production will ever be accurately tallied, as there were factories in Poland, East Germany, and Austria that produced unknown amounts of PCBs.[16]
Large-scale environmental contamination events
United States
Massachusetts
Pittsfield, in western Massachusetts, was home to the General Electric (GE) transformer and capacitor divisions, and electrical generating equipment built and repaired in Pittsfield powered the electrical utility grid throughout the nation. PCB-contaminated oil routinely migrated from GE’s 254-acre (1.03 km2) industrial plant located in the very center of the city to the surrounding groundwater, nearby Silver Lake, and to the Housatonic River, which flows through Massachusetts, Connecticut, and down to Long Island Sound.[17]
PCB-containing solid material was widely used as fill, including oxbows of the Housatonic River.[17] In the 1940s, GE launched a giveaway program in which locals could receive PCB-contaminated material to use as fill. The recipients were required to sign a letter stating they were receiving clean fill and releasing GE for any subsequent problems.[18] Beginning in 1997, the Massachusetts Department of Environmental Protection began evaluating over 700 suspect properties. As of 2010, over 250 properties had been found to have dangerous concentrations of PCBs in soil, and 180 properties had had contaminated soil removed.[citation needed]
New Bedford Harbor, which is a listed Superfund site,[19] contains some of the highest sediment concentrations in the marine environment.[20]
New York State
Between approximately 1947 and 1977, GE released up to 1,300,000 pounds (590,000 kg) of PCBs into the Hudson River.[21] The PCBs came from the company's two capacitor manufacturing plants at Hudson Falls and Fort Edward in New York State.[21]
In 1976, because of concern over continuing high levels of PCBs in local fish and other aquatic organisms, and the unacceptable risk to the health of consumers of such fish, the NYSDEC banned all fishing in the upper Hudson River, as well as commercial fishing of striped bass and several other species in the lower Hudson River,[21][22] and also issued advisories restricting the consumption of fish caught within a 20-mile (30 km) long segment of the Hudson River from Hudson Falls to Troy.[21][23]
There have been many programs of remediation work to reduce the PCB pollution. In 1984, approximately 200 miles (320 km) of the Hudson River was designated a Superfund site, and attempts to clean up the upper Hudson River began, including the removal in 1977-8 of 180,000 cubic yards (140,000 m3) of contaminated river sediments near Fort Edward.[22] In 1991, further PCB pollution was found at Bakers Falls, near the former GE Hudson Falls factory, and a program of remediation was started.[22] In August 1995, a 40-mile (64 km) reach of the upper Hudson was reopened to fishing, but only on a catch-and-release basis.[22] Removal of contaminated soil from Rogers Island was completed in December, 1999.[22] In 2002, the United States Environmental Protection Agency (EPA) announced a further 2,650,000 cubic yards (2,030,000 m3) of contaminated sediments in the upper Hudson River would be removed.
North Carolina
One of the largest PCB "spills" in American history occurred in the summer of 1978 when 31,000 gallons of PCB-contaminated oil were criminally and deliberately sprayed in 3-foot (0.91 m) swaths along the roadsides of some 240 miles (390 km) of North Carolina highway shoulders in 14 counties and at the Fort Bragg Army Base. The crime, known as "the midnight dumpings," lasted nearly 2 weeks, as drivers of a black-painted tanker truck drove down one side of rural Piedmont highways spraying their noxious liquid and then up the other side the following night.
Under the direction of Governor James B. Hunt, Jr., state officials then erected large, yellow warning signs along the contaminated highways that read: "CAUTION: PCB Chemical Spills Along Highway Shoulders."
The timing of the PCB crime was important. Toxic waste had become a national concern, and as the PCBs were literally being dumped along the roadsides in North Carolina, the Environmental Protection Agency's Toxic Substance Control Act, (TSCA), which specifically addressed the disposal of PCBs became effective (August 2, 1978). Also, simultaneously, residents of the contaminated Love Canal community were being evacuated from their homes because of an old toxic landfill. The pressure to build new, safer landfills was enormous. The rationale for the midnight dumpings, according to the state's logic, was that new EPA regulations were going to make disposing of PCBs more expensive, and companies might try to save money by disposing of toxic chemicals illegally; therefore, there was a pressing need to build convenient and affordable toxic waste landfills in North Carolina.
Within a couple of weeks of the crime, Robert Burns and his sons, Timothy and Randall, were arrested for dumping the PCBs along the roadsides. Burns was a business partner of Robert "Buck" Ward, Jr., of the Ward PCB Transformer Company, in Raleigh. Ward and Burns eventually did minimal time in jail for their crime because of federal charges; however, state charges were dropped.
While the Governor's plan was to pick up the roadside PCBs and to bury them in a landfill in rural Warren County, one of the most highly contaminated PCB counties, he faced a fierce opposition from residents who didn't want persistent, toxic PCBs to be buried in a landfill, which - according to new TSCA regulations - would be built only a few feet above their water table. For four years, residents of Warren County mounted an opposition to the PCB landfill that would eventuate in a six-week, non-violent direct-action campaign that would launch the environmental justice movement and that would, according to Johns Hopkins Professor Dr. Eileen McGurty, "transform environmentalism." see:Transforming Environmentalism: Warren County, PCBs, and the Origin of Environmental Justice.
Indiana
From the late 1950s through 1977, Westinghouse Electric used PCBs in the manufacture of capacitors in its Bloomington, Indiana plant. Reject capacitors were hauled and dumped in area salvage yards and landfills, including Bennett's Dump, Neal's Landfill and Lemon Lane Landfill.[24] Workers also dumped PCB oil down factory drains, which contaminated the city sewage treatment plant.[25] The City of Bloomington gave away the sludge to area farmers and gardeners, creating anywhere from 200 to 2000 sites, which remain unaddressed. Over 2 million pounds of PCBs were estimated to have been dumped in Monroe and Owen counties.[citation needed] Although federal and state authorities have been working on the sites' environmental remediation, many areas remain contaminated. Concerns have been raised regarding the removal of PCBs from the karst limestone topography, and regarding the possible disposal options. To date, the Westinghouse Bloomington PCB Superfund site case does not have a Remedial Investigation/Feasibility Study (RI/FS) and Record of Decision (ROD), although Westinghouse signed a US Department of Justice Consent Decree in 1985.[24] The 1985 consent decree required Westinghouse to construct an incinerator that would incinerate PCB-contaminated materials. Due to public opposition to the incinerator, however, the State of Indiana passed a number of laws that delayed and blocked its construction. The parties to the consent decree began to explore alternative remedies in 1994 for six of the main PCB contaminated sites.[24]
On 15 February 2008, Monroe County approved a plan to clean up the three remaining contaminated sites in the City of Bloomington, at a cost of $9.6 million to CBS Corp., the successor of Westinghouse.[26]
The Great Lakes
Much of the Great Lakes area is still heavily polluted with PCBs, despite extensive remediation work.[27] Locally-caught fresh water fish and shellfish are contaminated with PCBs, and their consumption is restricted.
From 1959 to 1971, Waukegan Harbor in Illinois, on Lake Michigan, was contaminated with PCBs discharged by the Outboard Marine Corporation.
Alabama
PCBs (manufactured through most of the 20th century) originating from Monsanto Chemical Company in Anniston, Alabama were dumped into Snow Creek, which then spread to Choccolocco Creek, then Logan Martin Lake. In the early 2000s, class action lawsuits (led, in at least one case, by the late Johnnie Cochran) were settled by local land owners, including those on Logan Martin Lake, and Lay Reservoir (downstream on the Coosa River), for the PCB pollution.
Today, the highest pollution levels remain concentrated in Snow and Choccolocco Creeks.[28] Concentrations in fish have declined and continue to decline over time; sediment disturbance, however, can resuspend the PCBs from the sediment back into the water column and food web.
South Carolina
From 1955 until 1977, the Sangamo Weston plant in Pickens, SC, used PCBs to manufacture capacitors, and dumped 400,000 pounds of PCB contaminated wastewater into the Twelve Mile Creek. In 1990, the EPA declared the 228 acres (0.92 km2) site of the capacitor plant, it's landfills and the polluted watershed, which stretches nearly 1,000 acres (4.0 km2) downstream to Lake Hartwell as a Superfund site. Two dams on the Twelve Mile Creek are to be removed and on Feb. 22, 2011 the first of two dams began to be dismantled. Some contaminated sediment is being removed from the site and hauled away, while other sediment is pumped into a series of settling ponds.[29][30]
Belgium
In 1999, the Dioxine affair caused serious trouble for the Belgian government, when PCBs were found in chicken and eggs.
Slovakia
The chemical plant Chemko in Strážske (east Slovakia) was an important producer of polychlorinated biphenyls for the former communist block (Comecon) until 1984. Chemko contaminated a large part of east Slovakia, especially the sediments of the Laborec river and reservoir Zemplínska šírava.[31][32]
Republic of Ireland
Main article: 2008 Irish pork crisisIn December 2008, a number of Irish news sources reported testing had revealed "extremely high"[33] levels of dioxins, by toxic equivalent, in pork products, ranging from 80 to 200 times the EU's upper safe limit of 1.5 pg WHO-TEQDFP/μg i.e. 0.12 to 0.3 parts per billion.[34][35]
Brendan Smith, the Minister for Agriculture, Fisheries and Food, stated the pork contamination was caused by PCB-contaminated feed that was used on 9 of Ireland's 400 pig farms, and only one feed supplier was involved.[34][36] Smith added that 38 beef farms also used the same contaminated feed, but those farms were quickly isolated and no contaminated beef entered the food chain.[37] While the contamination was limited to just 9 pig farms, the Irish government requested the immediate withdrawal and disposal of all pork-containing products produced in Ireland and purchased since 1 September 2008. This request for withdrawal of pork products was confirmed in a press release by the Food Safety Authority of Ireland on December 6.[38]
It is thought that the incident resulted from the contamination of fuel oil used in a drying burner at a single feed processor, with PCBs. The resulting combustion produced a highly toxic mixture of PCBs, dioxins and furans, which was included in the feed produced and subsequently fed to a large number of pigs. http://www.agriculture.gov.ie/media/migration/publications/2010/DioxinReport211209revised190110.pdf
Environmental transport and transformations
Due to their low vapour pressure, PCBs accumulate primarily in the hydrosphere, in the organic fraction of soil, and in organisms.[citation needed] Despite their hydrophobicity, the immense volume of water in the oceans is still capable of dissolving a significant quantity of PCBs.
However, a small volume of PCBs has been detected throughout the atmosphere, from the most urbanized areas that are the centers for PCB pollution, to regions north of the Arctic Circle. While the hydrosphere is the main reservoir, the atmosphere serves as the primary route for global transport of PCBs, particularly for those congeners with one to four chlorine atoms.
Atmospheric concentrations of PCBs tend to be lowest in rural areas, where they are typically in the picogram per cubic meter range, higher in suburban and urban areas, and highest in city centres, where they can reach 1 ng/m³ or more. In Milwaukee, an atmospheric concentration of 1.9 ng/m³ has been measured, and this source alone was estimated to account for 120 kg/year of PCBs entering Lake Michigan.[39] Concentrations as high as 35 ng/m³, 10 times higher than the EPA guideline limit of 3.4 ng/m³, have been found inside some houses in the U.S.[8]
Volatilization of PCBs in soil was thought to be the primary source of PCBs in the atmosphere, but recent research suggests ventilation of PCB-contaminated indoor air from buildings is the primary source of PCB contamination in the atmosphere.[40]
In the atmosphere, PCBs may be degraded by hydroxyl radicals, or directly by photolysis of carbon-chlorine bonds (even if this is a less important process).
In biosphere, PCBs can be degraded by either bacteria or eukariotes, but the speed of the reaction depends on both the number and the disposition of chlorine atoms in the molecule: less substituted, meta- or para- substituted PCBs undergo biodegradation faster than more substituted congeners.
In bacteria, PCBs may be dechlorinated through reductive dechlorination, or oxidized by dioxygenase enzyme.
In eukariotes, PCBs may be oxidized by the cytochrome P450 enzyme.
Health effects
The toxicity of PCBs had been known since before its first commercial production through research done by producing companies themselves in the 1930s; however, these conclusions were dismissed as negligible.
The toxicity of PCBs to animals was first noticed in the 1970s, when emaciated seabird corpses with very high PCB body burdens washed up on beaches. Since seabirds may die far out at sea and still wash ashore, the true sources of the PCBs were unknown. Where they were found was not a reliable indicator of where they had died.
The toxicity of PCBs varies considerably among congeners. The coplanar PCBs, known as nonortho PCBs because they are not substituted at the ring positions ortho to (next to) the other ring, (i.e. PCBs 77, 126, 169, etc.), tend to have dioxin-like properties, and generally are among the most toxic congeners. Because PCBs are almost invariably found in complex mixtures, the concept of toxic equivalency factors (TEFs) has been developed to facilitate risk assessment and regulatory control, where more toxic PCB congeners are assigned higher TEF values on a scale from 0 to 1. One of the most toxic compounds known, 2,3,7,8-tetrachlorodibenzo[p]dioxin, is assigned a TEF of 1.[41]
PCBs also have shown toxic and mutagenic effects by interfering with hormones in the body. PCBs, depending on the specific congener, have been shown to both inhibit and imitate estradiol, the main sex hormone in females. Imitation of the estrogen compound can feed estrogen-dependent breast cancer cells, and possibly cause other cancers, such as uterine or cervical. Inhibition of estradiol can lead to serious developmental problems for both males and females, including sexual, skeletal, and mental development issues.
Exposure and excretion
Individuals can be exposed to PCBs through breathing in contaminated air, consuming contaminated food, and by skin contact with old electrical equipment that contain PCBs. Once exposed, some PCBs may change to other chemicals inside the body. These chemicals or unchanged PCBs can be excreted in feces or may remain in a person's body fat or other organs for months. PCBs may also collect in milk fat and be transmitted to infants through breast-feeding.[42]
Signs and symptoms
- Humans
- The most commonly observed health effects in people exposed to extremely high levels of PCBs are skin conditions, such as chloracne and rashes, but these were known to be symptoms of acute systemic poisoning dating back to 1922. Studies in workers exposed to PCBs have shown changes in blood and urine that may indicate liver damage. In Japan in 1968, 280 kg of PCB-contaminated rice bran oil was used as chicken feed, resulting in a mass poisoning, known as Yushō disease, in over 14,000 people.[43] Common symptoms included dermal and ocular lesions, irregular menstrual cycles and lowered immune responses.[44][45][46] Other symptoms included fatigue, headaches, coughs, and unusual skin sores.[47] Additionally, in children, there were reports of poor cognitive development.[44][46][47]
- There have also been studies of the health effects of PCBs in the general population and in children of mothers who were exposed to PCBs.
- Animals
- Animals that eat PCB-contaminated food even for short periods of time suffer liver damage and may die. In 1968 in Japan, 400,000 birds died after eating poultry feed that was contaminated with PCBs.[43] Animals that ingest smaller amounts of PCBs in food over several weeks or months develop various health effects, including anemia; acne-like skin conditions (chloracne); and liver, stomach, and thyroid gland injuries (including hepatocarcinoma). Other effects of PCBs in animals include changes in the immune system, behavioral alterations, and impaired reproduction. PCBs are not known to cause birth defects in humans, although those that have dioxin-like activity are known to cause a variety of teratogenic effects in animals.
- Effects during pregnancy/breastfeeding
- Women who were exposed to relatively high levels of PCBs in the workplace or ate large amounts of fish contaminated with PCBs had babies that weighed slightly less than babies from women who did not have these exposures. Babies born to women who ate PCB-contaminated fish also showed abnormal responses in tests of infant behavior. Some of these behaviors, such as problems with motor skills and a decrease in short-term memory, lasted for several years. Other studies suggest that the immune system was affected in children born to and nursed by mothers exposed to increased levels of PCBs. The most likely way infants will be exposed to PCBs is from breast milk. Transplacental transfers of PCBs were also reported.
- Studies have shown PCBs alter estrogen levels in the body and contribute to reproduction problems. In the womb, males can be feminised or the baby may be intersex, neither a male nor a female. Also, both sets of reproductive organs may develop. More instances of this are being reported. Biological magnification of PCBs has also led to polar bears and whales that have both male and female sex organs and males that cannot reproduce. This effect is known as endocrine disruption. Endocrine disrupting chemicals (EDCs) pose a serious threat to reproduction in top-level predators.
Cancer link
A few studies of workers indicate PCBs were associated with specific kinds of cancer in humans, such as cancer of the liver and biliary tract. PCBs also have been shown to mimic the action of estrogen in breast cancer cells and can enhance breast carcinogenesis.[48] Rats that ate food containing high levels of PCBs for two years developed liver cancer. The Department of Health and Human Services (DHHS) has concluded PCBs may reasonably be anticipated to be carcinogens. The EPA and the International Agency for Research on Cancer (IARC) have determined that PCBs are probably carcinogenic to humans. PCBs are also classified as probable human carcinogens by the National Cancer Institute, World Health Organization, and the Agency for Toxic Substances and Disease Registry. Recent research by the National Toxicology Program has confirmed PCB126 (Technical Report 520) and a binary mixture of PCB126 and PCB153 (Technical Report 531) are carcinogens.
Mechanism of action
As discussed, PCBs exhibit a wide range of toxic effects. These effects may vary depending on the specific PCB. Similar to dioxin, toxicity of coplanar PCBs and mono-ortho-PCBs are thought to be primarily mediated via binding to aryl hydrocarbon receptor (AhR).[49][50] Because AhR is a transcription factor, abnormal activation may disrupt cell function by altering the transcription of genes. The concept of toxic equivalency factors (TEF) is based on the ability of a PCB to activate AhR.
However, not all effects may be mediated by the AhR receptor, and PCBs do not alter estrogen concentrations to the same degree as other ligands of the AhR receptor, such as PCDD and PCDF, possibly reflecting the reduced potency of PCBs to induce CYP1A1 and CYP1B1.[51] Examples of other actions of PCBs include di-ortho-substituted non-coplanar PCBs interfering with intracellular signal transduction dependent on calcium; this may lead to neurotoxicity.[52] Ortho-PCBs may disrupt thyroid hormone transport by binding to transthyretin.[53]
Congeners that show estrogenic effects bind to the 17 β estradiol receptor. The resulting complex is allowed to enter the nucleus and bind to specific segments of DNA, where it regulates transcription of certain segments of DNA into mRNA. This transcription may not be regulated properly, since it is being controlled by PCBs and not estradiol.
Containment
Because of its difficult containment, many buildings (at least in the U.S.A.) with known high PCB dangers have been evacuated and shut down. In many states, including California, laws require any building with such dangers to be sealed and locked, with large warning signs on every entrance point indicating a PCB presence and also a notice to indicate the presence of chemicals known to cause cancer, health problems or reproductive harm[citation needed]. Until a safe solution can be well established, many of these buildings remain undemolished and sealed. Some forms of containment other than building closure and lockdown are below.
Landfill – Large quantities of PCBs have been placed in landfill sites, mainly in the form of transformers and capacitors. Many municipal sites are not designed to contain these pollutants and PCBs are able to escape into the atmosphere or ground water. No emissions above background are seen if the landfill is designed correctly.
Methods of destruction
These can be separated into three distinct categories: physical, microbial, and chemical destruction.
Physical
Incineration – Although PCBs do not ignite themselves, they can be combusted under extreme and carefully controlled conditions. The current regulations require that PCBs are burnt at a temperature of 1200 °C for at least two seconds, in the presence of fuel oil and excess oxygen.[54] A lack of oxygen can result in the formation of PCDDs, PCDFs and dioxins, or the incomplete destruction of the PCBs. Such specific conditions mean that it is extremely expensive to destroy PCBs on a tonnage scale, and it can only be used on PCB-containing equipment and contaminated liquid. This method is not suitable for the decontamination of affected soils.
Ultrasound – In a similar process to combustion, high power ultrasonic waves are applied to water, generating cavitation bubbles. These then implode or fragment, creating microregions of extreme pressures and temperatures where the PCBs are destroyed. Water is thought to undergo thermolysis, oxidising the PCBs to CO, CO2 and hydrocarbons such as biphenyl, and releasing chlorine. The scope of this method is limited to those congeners that are the most water-soluble, those isomers with the least chlorine substitution.[citation needed]
Irradiation – If a deoxygenated mixture of PCBs in isopropanol or mineral oil is subjected to irradiation with gamma rays then the PCBs will be dechlorinated to form biphenyl and inorganic chloride. The reaction works best in isopropanol if potassium hydroxide (caustic potash) is added. Solvated electrons are thought to be responsible for the reaction. If oxygen, nitrous oxide, sulfur hexafluoride, or nitrobenzene is present in the mixture then the reaction rate is reduced. This work has been done recently in the US often with used nuclear fuel as the radiation source.[55][56]
Pyrolysis – Destruction of PCBs with pyrolysis using plasma arc processes, like incineration, uses heat. However, unlike incineration, there is no combustion. The long-chain molecules are broken with extreme temperature provided by an electric arc in an inert environment. Adequate post-pyrolysis treatment of the resultant products is required in order to prevent the risk of back reactions.[citation needed]
Microbial
Much recent work has centered on the study of micro-organisms that are able to decompose PCBs. In general, these organisms work in one of two ways: Either they use the PCB as a carbon source or destruction takes place through reductive dechlorination, with the replacement of chlorine with hydrogen on the biphenyl skeleton. However, there are significant problems with this approach. First, these microbes tend to be highly selective in their dechlorination, with lower chlorinated biphenyls being readily transformed, and with preference to dechlorination in the para and meta positions. Second, microbial dechlorination tends to be rather slow-acting on PCB as a soil contaminant in comparison to other methods. Last, while microbes work well in laboratory conditions, there is often a problem in transferring a successful laboratory strain to a natural system. This is because the microbes can access other sources of carbon, which they decompose in preference to PCBs.[citation needed]
Further recent developments have focused on testing enzymes and vitamins extracted from microbes that show PCB activity. Especially promising seems to be the use of vitamin B12, in which a cobalt ion is in oxidation state (III) under normal redox conditions. Using titanium (III) citrate as a strong reductant converts the cobalt from Co(III) to Co(I), giving a new vitamin known as B12s, which is a powerful nucleophile and reducing catalyst. This can then be used on PCBs, which it dechlorinates in a rapid and selective manner.[57]
Chemical
Many chemical methods[dead link] are available to destroy or reduce the toxicity of PCBs.
Nucleophilic aromatic substitution is a method of destroying low-concentration PCB mixtures in oils, such as transformer oil. Substitution of chlorine by polyethylene glycols occurs in under two hours under a blanket of nitrogen, to prevent oxidation of the oil, to produce aryl polyglycols, which are insoluble in the oil and precipitate out.
Between 700 and 925 °C, H2 cleaves the carbon-chlorine bond, and cleaves the biphenyl nucleus into benzene, yielding HCl without a catalyst. This can be performed at lower temperatures with a copper catalyst, and to yield biphenyl. However, since both of these routes require an atmosphere of hydrogen gas and relatively high temperatures, they are prohibitively expensive.
Reaction with highly electropositive metals, or strong reducing agents such as sodium naphthalide, in aprotic solvents results in a transfer of electrons to the PCB, the expulsion of a chloride ion, and a coupling of the PCBs. This is analogous to the Wurtz reaction for coupling halogenoalkanes. The effect is to polymerise many molecules, thereby reducing the volatility, solubility, and toxicity of the mixture. This methodology is most successful on low-strength PCB mixtures and can also be performed electrochemically in a partly aqueous bicontinuous microemulsion.
The solution photochemistry of PCBs is based on the transfer of an electron to a photochemically excited PCB from a species such as an amine, to give a radical anion. This either expels a chloride ion and the resulting aryl radical extracts a hydrogen atom from the solvent or immediately becomes protonated, leading to the loss of a chlorine atom. It is useful only for water-soluble PCBs.
The major pathway for atmospheric destruction of PCBs is via attack by OH radicals. Direct photolysis can occur in the upper atmosphere, but the ultraviolet wavelengths necessary to excite PCBs are shielded from the troposphere by the ozone layer. It has, however, been shown that higher wavelengths of light (> 300 nm) can degrade PCBs in the presence of a photosensitizer, such as acetone.
The Schwartz reaction is the subject of much study, and has significant benefits over other routes. It is advantageous since it proceeds via a reductive process, and thus yields no dioxins through oxidation. The proposed reaction scheme involves the electron transfer from a titanium (III) organometallic species to form a radical anion on the PCB molecule which expels chlorine to eventually form the relatively non-toxic biphenyl.
PCB info table
For a complete list of PCB congeners, see PCB Congener List. Note that biphenyl, while not technically a PCB congener due to its lack of chlorine substituents, is still typically included in the literature.
PCB Homolog CASRN Cl Substituents Number of Congeners Biphenyl 92-52-4 0 1 Monochlorobiphenyl 27323-18-8 1 3 Dichlorobiphenyl 25512-42-9 2 12 Trichlorobiphenyl 25323-68-6 3 24 Tetrachlorobiphenyl 26914-33-0 4 42 Pentachlorobiphenyl 25429-29-2 5 46 Hexachlorobiphenyl 26601-64-9 6 42 Heptachlorobiphenyl 28655-71-2 7 24 Octachlorobiphenyl 55722-26-4 8 12 Nonachlorobiphenyl 53742-07-7 9 3 Decachlorobiphenyl 2051-24-3 10 1 See also
- Bay mud
- GE
- Monsanto
- Organochlorine compound
- Polybrominated biphenyls
- Zodiac, a novel by Neal Stephenson which involves PCBs and their impact on the environment.
Notes
- ^ a b Porta M, Zumeta E. Implementing the Stockholm treaty on POPs [Editorial]. Occupational & Environmental Medicine 2002; 59: 651-652. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1740221/pdf/v059p00651.pdf
- ^ a b c d e f g h i j k "Proceedings of the Subregional Awareness Raising Workshop on Persistent Organic Pollutants (POPs), Bangkok, Thailand". United Nations Environment Programme. November 25-28th, 1997. http://www.chem.unep.ch/pops/POPs_Inc/proceedings/bangkok/FIEDLER1.html. Retrieved 2007-12-11.
- ^ Amy Boate, Greg Deleersnyder, Jill Howarth, Anita Mirabelli, and Leanne Peck (2004). "Chemistry of PCBs". http://wvlc.uwaterloo.ca/biology447/modules/intro/assignments/Introduction2a.htm. Retrieved 2007-11-07.
- ^ a b (PDF) Identifying PCB-Containing Capacitors. Australian and New Zealand Environment and Conservation Council (ANZECC). 1997. pp. 4–5. ISBN 0 642 54507 3. http://www.environment.gov.au/settlements/publications/chemicals/scheduled-waste/pubs/pcbid.pdf. Retrieved 2007-07-07.
- ^ Gerhard Winneke, Albert Bucholski, Birger Heinzow, Ursula Kramer, Eberhard Schmidt, Jens Walkowiak, Jorg-Andreas Wiener, Hans-Joachim Steingruber, Developmental neurotoxicity of polychlorinated biphenyls (PCBS): cognitive and psychomotor functions in 7-month old children, Toxicology Letters, Volumes 102-103, 28 December 1998, Pages 423-428, ISSN 0378-4274, DOI: 10.1016/S0378-4274(98)00334-8. (http://www.sciencedirect.com/science/article/B6TCR-3VH7YFG-2C/2/8e8b30dfae2aec982c155a132e35c6fa) Keywords: PCB; Children; Mental development; Psychomotor functions; Confounding; Risk assessment
- ^ "Brand names of PCBs — What are PCBs?". Japan Offspring Fund / Center for Marine Environmental Studies (CMES), Ehime University, Japan. 2003. http://tabemono.info/report/former/pcd/2/2_2/e_1.html. Retrieved 2008-02-11.
- ^ Godish, T. (2001). Indoor environmental quality. (1 ed., pp. 110-130). Boca Raton, Florida: Lewis Publishers.
- ^ a b c Rudel, R A, Seryak, L M, and Brody, J G (2008). "PCB-containing wood floor finish is a likely source of elevated PCBs in resident's blood, household air and dust: a case study of exposure". Environmental Health 7: 2. doi:10.1186/1476-069X-7-2. PMC 2267460. PMID 18201376. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2267460.
- ^ Karlyn Black Kaley, Jim Carlisle, David Siegel, Julio Salinas (October 2006) (pdf). Health Concerns and Environmental Issues with PVC-Containing Building Materials in Green Buildings. Integrated Waste Management Board, California Environmental Protection Agency, USA. pp. 11. http://www.ciwmb.ca.gov/publications/GreenBuilding/43106016.pdf. Retrieved 2007-08-03.
- ^ Drinker, C.K., M.F. Warren, and G.A. Bennet (1937). "The problem of possible systemic effects from certain chlorinated hydrocarbons". Journal of Industrial Hygiene and Toxicology 19 (7): 283.
- ^ Butler, D.A. (2005). "The early history of scientific and medical research on agent orange" (PDF). Brooklyn Journal of Law and Policy 13 (2): 527. http://brooklaw.edu/students/journals/bjlp/jlp13ii_butler.pdf.
- ^ Brown, R. M. (1947). "The toxicity of the 'Arochlors'". Chemist-Analyst 36: 33. http://chemport.cas.org/cgi-bin/sdcgi?APP=ftslink&action=reflink&origin=npg&version=1.0&coi=1:CAS:528:DyaH2sXjs1WntA%3D%3D&pissn=0028-0836&pyear=1967&md5=58285ba11405fbe2e87bf46712d4f63d.
- ^ S. Jensen (1966). "Report of a new chemical hazard". New Sci. 32: 612.
- ^ EPA.gov
- ^ "Guidance on municipal waste strategies, Section 5.12 Equipment, which contains low volumes of PCBs" (PDF). UK Department of the Environment, Transport and the Regions. 2001. p. 17. http://www.defra.gov.uk/environment/waste/management/guidance/mwms/pdf/mwms.pdf. Retrieved 2008-01-29.
- ^ Breivik K, Sweetman A, Pacyna JM, Jones KC (2002). "Towards a global historical emission inventory for selected PCB congeners - a mass balance approach 1. Global production and consumption". The Science of the Total Environment 290 (1–3): 181–198. doi:10.1016/S0048-9697(01)01075-0. PMID 12083709.
- ^ a b "GE/Housatonic River Site in New England: Site History and Description". USEPA. http://www.epa.gov/NE/ge/sitehistory.html. Retrieved 18 July 2010.
- ^ "GOOD THINGS TO LIFE". Blue Hill Films. http://www.bluehillfilms.com/?page_id=7. Retrieved 18 July 2010.
- ^ USEPA NPL Factsheet-NEW BEDFORD SITE
- ^ Case: New Bedford Harbor, MA, NOAA
- ^ a b c d "Hudson River PCBs — Background and Site Information". United States Environmental Protection Agency. http://www.epa.gov/hudson/background.htm. Retrieved 2007-12-31.
- ^ a b c d e "Hudson River PCBs" (PDF). United States Environmental Protection Agency. 2007-06-27. http://www.epa.gov/Region2/superfund/npl/0202229c.pdf. Retrieved 2007-12-31.
- ^ "Hudson River Virtual Tour — Chapter 9: PCB Pollution in the Hudson". NYSDEC. http://www.dec.ny.us/lands/25974.html. Retrieved 2007-12-31.
- ^ a b c "US EPA Region 5 Superfund". http://www.epa.gov/R5Super/npl/indiana/IND006418651.htm. Retrieved 2009-09-01.
- ^ "Westinghouse/ABB Plant Facility". 2006. http://copa.org/abb.html. Retrieved 2010-02-24.
- ^ "Monroe Co. approves PCB clean up". IndyStar.com. Archived from the original on 2008-02-18. http://www.webcitation.org/query?url=http%3A%2F%2Fwww.indystar.com%2Fapps%2Fpbcs.dll%2Farticle%3FAID%3D%2F20080218%2FLOCAL%2F802180394&date=2008-02-18. Retrieved 2008-02-18.
- ^ Bette Hileman (1988-02-08). "The Great Lakes cleanup effort". Chemistry and Engineering 8: 22–39.
- ^ Rypel, A.L., D.R. Bayne, J.B. Mitchell and R.H. Findlay. 2007. Variations in PCB concentrations between genders of six warmwater fish species in Lake Logan Martin, Alabama, U.S.A., Chemosphere, 68: 1707-1715
- ^ Sangamo Weston, Inc./Twelve-Mile Creek/Lake Hartwell PCB Contamination, USEPA
- ^ Dam Demolition Begins On Twelve Mile River, WYFF News 4, February 22, 2011.
- ^ Viete, čo doma dýchate?, Život, 33/2009
- ^ Toxické záťaže - Chemko Strážske
- ^ Alan Reilly of the Food Safety Authority of Ireland as quoted in The Irish Time Monday, December 8, 2008
- ^ a b Food body to meet on pork recall BBC News
- ^ Commission Regulation (EC) No 1881/2006 Maximum levels for certain contaminants in foodstuffs
- ^ Food Minister criticises pork monitoring RTÉ News
- ^ "Q&A: The recall of Irish pork". BBC. 2008-12-07. http://news.bbc.co.uk/2/hi/uk_news/7769893.stm. Retrieved 2008-12-08.
- ^ FSAI.ie
- ^ Wethington, D M III and Hornbuckle, K C (2005). "Milwaukee, WI, as a Source of Atmospheric PCBs to Lake Michigan". Environmental Science 39 (1): 57–63. doi:10.1021/es048902d.
- ^ Jamshidi A, Hunter S, Hazrati S, and Harrad S (2007). "Concentrations and Chiral Signatures of Polychlorinated Biphenyls in Outdoor and Indoor Air and Soil in a Major UK. Conurbation". Environmental Science Technology 41 (7): 2153–2158. doi:10.1021/es062218c. PMID 17438756.
- ^ Van den Berg M (December 1998). "Toxic Equivalency Factors (TEFs) for PCBs, PCDDs, PCDFs for Humans and Wildlife". Environ Health Perspect. (Brogan &) 106 (12): 775–792. doi:10.2307/3434121. JSTOR 3434121. PMC 1533232. PMID 9831538. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1533232.
- ^ [1]
- ^ a b "Contamination of rice bran oil with PCB used as the heating medium by leakage through penetration holes at the heating coil tube in deodorization chamber". Hatamura Institute for the Advancement of Technology. http://www.sozogaku.com/fkd/en/cfen/CB1056031.html. Retrieved 2007-12-11.
- ^ a b Polychlorinated biphenyls, polychlorinated dibenzo...[Environ Res. 2001] - PubMed Result
- ^ Disease ID 8326 at NIH's Office of Rare Diseases
- ^ a b PCB Baby Studies Part 2
- ^ a b Environmental Diseases from A to Z
- ^ Staff Profile:Dr Philippa Darbre
- ^ Safe S; Hutzinger, Otto (1984). "Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanism of action". Crit. Rev. Toxicol. 13 (4): 319–95. doi:10.3109/10408448409023762. PMID 6091997.
- ^ Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W (1985). "PCBs: structure-function relationships and mechanism of action". Environ. Health Perspect. (Brogan &) 60: 47–56. doi:10.2307/3429944. JSTOR 3429944. PMC 1568577. PMID 2992927. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1568577.
- ^ Body burdens of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls and their relations to estrogen metabolism in pregnant women. Wang SL, Chang YC, Chao HR, Li CM, Li LA, Lin LY, Päpke O. Environ Health Perspect. 2006 May;114(5):740-5.
- ^ Simon T, Britt JK, James RC (2007). "Development of a neurotoxic equivalence scheme of relative potency for assessing the risk of PCB mixtures". Regulatory toxicology and pharmacology : RTP 48 (2): 148–70. doi:10.1016/j.yrtph.2007.03.005. PMID 17475378.
- ^ Chauhan KR, Kodavanti PR, McKinney JD (2000). "Assessing the role of ortho-substitution on polychlorinated biphenyl binding to transthyretin, a thyroxine transport protein". Toxicol. Appl. Pharmacol. 162 (1): 10–21. doi:10.1006/taap.1999.8826. PMID 10631123.
- ^ [2]
- ^ OSTI.gov
- ^ Patentstorm.us
- ^ Woods SL, Trobaugh DJ (1999). "Polychlorinated Biphenyl Reductive Dechlorination by Vitamin B12s: Thermodynamics and Regiospecificity". Environ. Sci. Technol. 33 (6): 857–863. doi:10.1021/es9804823.
External links
- ATSDR Toxicological Profile U.S. Department of Health and Human Services
- IARC PCB Monograph
- US EPA PCB Homepage
- National Toxicology Program technical reports
- Polychlorinated Byphenyls: Human Health Aspects by the WHO
- Case study of plasma arc process for the destruction of PCBs
- Current Intelligence Bulletin 7: Polychlorinated (PCBs) -- NIOSH/CDC
- It's Your Health - PCBs (Health Canada)
Health issues of plastics and Polyhalogenated compounds (PHCs) Plasticizers: Phthalates Miscellaneous plasticizers Monomers Bisphenol A (BPA, in Polycarbonates) · Vinyl chloride (in PVC)Miscellaneous additives incl. PHCs Health issues Miscellanea PVC · Plastic recycling · Plastic bottle · Vinyl chloride · Dioxins · Polystyrene · Styrofoam · PTFE (Teflon) · California Proposition 65 · List of environmental health hazards · Persistent organic pollutant · European REACH regulation · Japan Toxic Substances Law · Toxic Substances Control ActCategories:- Organochlorides
- Flame retardants
- Endocrine disruptors
- Hazardous air pollutants
- IARC Group 2A carcinogens
- Persistent organic pollutants
- Soil contamination
- Synthetic materials
Wikimedia Foundation. 2010.