- Dioxygenase
-
Dioxygenase 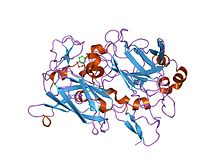
crystal structure of acinetobacter sp. adp1 protocatechuate 3,4-dioxygenase in complex with 3,4-dihydroxybenzoate Identifiers Symbol Dioxygenase_C Pfam PF00775 Pfam clan CL0287 InterPro IPR000627 PROSITE PDOC00079 SCOP 2pcd Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Catechol dioxygenase N terminus 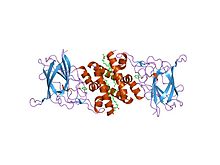
crystal structure of 4-chlorocatechol 1,2-dioxygenase from rhodococcus opacus 1cp Identifiers Symbol Dioxygenase_N Pfam PF04444 InterPro IPR007535 SCOP 1dlm Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary In molecular biology, a dioxygenase is an enzyme which catalyses the incorporation of both atoms of molecular oxygen into substrates using a variety of reaction mechanisms. Cleavage of aromatic rings is one of the most important functions of dioxygenases, which play key roles in the degradation of aromatic compounds. The substrates of ring-cleavage dioxygenases can be classified into two groups according to the mode of scission of the aromatic ring. Intradiol enzymes use a non-haem Fe(III) to cleave the aromatic ring between two hydroxyl groups (ortho-cleavage), whereas extradiol enzymes use a non-haem Fe(II) to cleave the aromatic ring between a hydroxylated carbon and an adjacent non-hydroxylated carbon (meta-cleavage).[1] These two subfamilies differ in sequence, structural fold, iron ligands, and the orientation of second sphere active site amino acid residues.
Enzymes that belong to the intradiol family include catechol 1,2-dioxygenase (1,2-CTD) EC 1.3.11.1; protocatechuate 3,4-dioxygenase (3,4-PCD) EC 1.3.11.3 and chlorocatechol 1,2-dioxygenase EC 1.3.11.1[2]
Enzymes that belong to the extradiol class II family include catechol 2,3-dioxygenase (2,3-CTD) EC 1.3.11.2 and biphenyl-2,3-diol 1,2-dioxygenase (BphC) EC 1.3.11.39.
References
- ^ Broderick JB (1999). "Catechol dioxygenases". Essays Biochem. 34: 173–89. PMID 10730195.
- ^ Ferraroni M, Solyanikova IP, Kolomytseva MP, Scozzafava A, Golovleva L, Briganti F (June 2004). "Crystal structure of 4-chlorocatechol 1,2-dioxygenase from the chlorophenol-utilizing gram-positive Rhodococcus opacus 1CP". J. Biol. Chem. 279 (26): 27646–55. doi:10.1074/jbc.M401692200. PMID 15060064.
This article includes text from the public domain Pfam and InterPro IPR000627
Categories:- Protein domains
Wikimedia Foundation. 2010.
