- Bicalutamide
-
Bicalutamide 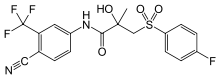
Systematic (IUPAC) name N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide Clinical data Trade names Casodex AHFS/Drugs.com monograph MedlinePlus a697047 Pregnancy cat. X(US) Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Bioavailability well absorbed Protein binding 96% Metabolism hepatic Half-life 5.8 days Identifiers CAS number 90357-06-5 
ATC code L02BB03 PubChem CID 2375 DrugBank APRD00042 ChemSpider 2284 
UNII A0Z3NAU9DP 
KEGG D00961 
ChEMBL CHEMBL63560 
Chemical data Formula C18H14F4N2O4S Mol. mass 430.373 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Bicalutamide (marketed as Casodex, Cosudex, Calutide, Kalumid) is an oral non-steroidal anti-androgen used in the treatment of prostate cancer[1][2][3] and hirsutism.[4] It was first launched in 1995 as a combination treatment (with surgical or medical castration) for advanced prostate cancer and subsequently launched as monotherapy for the treatment of earlier stages of the disease.
Bicalutamide is marketed by AstraZeneca with the brand names Casodex and Cosudex. It is recommended 50 mg once daily in combination with a luteinizing hormone-releasing hormone analogue or surgical castration.[5]
Contents
Description and mechanism of action
Bicalutamide is an oral non-steroidal anti-androgen with the empirical formula C18H14F4N2O4S and is an off-white powder that is practically insoluble in water.[6]
Bicalutamide acts as a pure anti-androgen by binding to the androgen receptor (AR) and preventing the activation of the AR and subsequent upregulation of androgen responsive genes by androgenic hormones.[7][8] In addition, bicalutamide accelerates the degradation of the androgen receptor.[9] Bicalutamide has been used as a molecular template for the design of selective androgen receptor modulators (SARMs) such as Andarine[10] and Ostarine.
Indications and use
Bicalutamide is indicated for the treatment of stage D2 metastatic prostate cancer in combination with a luteinizing hormone-releasing hormone analogue or as a monotherapy.[11] It has also been used in clinical trials for ovarian cancer.[12] It has also been used in combination with castration.[13]
Most advanced prostate cancer patients eventually become resistant to antiandrogen including bicalutamide therapy.[14]
Contraindications and precautions
Bicalutamide is contraindicated in patients who have shown a hypersensitivity reaction to its use.[6] Bicalutamide is a teratogen and must not be handled by females who are or may become pregnant. It is known to cause fetal harm. pg8
Adverse reactions
Adverse reactions include reproductive system and breast disorders, breast tenderness, gynaecomastia, hot flushes, gastrointestinal disorders, diarrhoea, nausea, hepatic changes (elevated levels of transaminases, jaundice), asthenia and pruritus.[15][16]
Physiology
Bicalutamide blocks androgen receptors. This prevents testosterone and other androgens from binding to the receptors. Bicalutamide may cause sexual difficulties and a decline in sperm count. Nevertheless bicalutamide monotherapy appears to have minimal effect on sexual activity.[17]
Blockade of androgens receptors by bicalutamide in the brain will eliminate the negative feedback loop of testosterone on the release of luteinizing hormone (LH). This in turn will lead to a dramatic increase in testosterone and estrogen levels.[18] Bicalutamide treatment will block the effects of rising testosterone levels, but the effect of rising estrogen levels will remain unopposed and lead to feminizing effects, the most notable one being gynecomastia, which is often painful.[19]
If bicalutamide is combined with an LHRH agonist or surgical castration then the elevation of estrogen levels will be prevented and the risks of excessive estrogen will be reduced. However, since both testosterone and estrogens are essential for normal bone metabolism, reducing the anabolic bone effects of both androgens (which increase bone formation by stimulating osteoblasts)[20] and estrogens (which reduce bone resorption by inhibiting osteoclasts)[21] will increase bone loss and promote osteoporosis.[22]
References
- ^ Schellhammer PF (September 2002). "An evaluation of bicalutamide in the treatment of prostate cancer". Expert Opin Pharmacother 3 (9): 1313–28. doi:10.1517/14656566.3.9.1313. PMID 12186624.
- ^ Fradet Y; James, Nick; Maher, Jane (February 2004). "Bicalutamide (Casodex) in the treatment of prostate cancer". Expert Rev Anticancer Ther 4 (1): 37–48. doi:10.1586/14737140.4.5.S37. PMID 14748655.
- ^ See WA, Tyrrell CJ (August 2006). "The addition of bicalutamide 150 mg to radiotherapy significantly improves overall survival in men with locally advanced prostate cancer". Journal of cancer research and clinical oncology 132 Suppl 1: S7–16. doi:10.1007/s00432-006-0132-6. PMID 16896884.
- ^ Müderris II, Bayram F, Ozçelik B, Güven M (February 2002). "New alternative treatment in hirsutism: bicalutamide 25 mg/day". Gynecol. Endocrinol. 16 (1): 63–6. doi:10.1080/713602986. PMID 11915584.
- ^ Klotz L (May 2006). "Combined androgen blockade: an update". Urol. Clin. North Am. 33 (2): 161–6, v–vi. doi:10.1016/j.ucl.2005.12.001. PMID 16631454.
- ^ a b "Casodex product insert" (PDF). www1.astrazeneca-us.com. 2006-03-01. http://www1.astrazeneca-us.com/pi/casodex.pdf/. Retrieved 2008-12-27.
- ^ Furr BJ (1996). "The development of Casodex (bicalutamide): preclinical studies". Eur. Urol. 29 Suppl 2: 83–95. PMID 8717469.
- ^ Furr BJ, Tucker H (January 1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology 47 (1A Suppl): 13–25; discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- ^ Waller AS, Sharrard RM, Berthon P, Maitland NJ (June 2000). "Androgen receptor localisation and turnover in human prostate epithelium treated with the antiandrogen, casodex". J. Mol. Endocrinol. 24 (3): 339–51. doi:10.1677/jme.0.0240339. PMID 10828827.
- ^ Chen J, Kim J, Dalton JT (June 2005). "Discovery and therapeutic promise of selective androgen receptor modulators". Mol. Interv. 5 (3): 173–88. doi:10.1124/mi.5.3.7. PMC 2072877. PMID 15994457. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2072877.
- ^ Schellhammer PF, Sharifi R, Block NL, Soloway MS, Venner PM, Patterson AL, Sarosdy MF, Vogelzang NJ, Schellenger JJ, Kolvenbag GJ (September 1997). "Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group". Urology 50 (3): 330–6. doi:10.1016/S0090-4295(97)00279-3. PMID 9301693.
- ^ Levine D, Park K, Juretzka M, Esch J, Hensley M, Aghajanian C, Lewin S, Konner J, Derosa F, Spriggs D, Iasonos A, Sabbatini P (December 2007). "A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission". Cancer 110 (11): 2448–56. doi:10.1002/cncr.23072. PMID 17918264.
- ^ Klotz L, Schellhammer P (March 2005). "Combined androgen blockade: the case for bicalutamide". Clin Prostate Cancer 3 (4): 215–9. PMID 15882477.
- ^ Balk SP (September 2002). "Androgen receptor as a target in androgen-independent prostate cancer". Urology 60 (3 Suppl 1): 132–8; discussion 138–9. doi:10.1016/S0090-4295(02)01593-5. PMID 12231070.
- ^ Lunglmayr G (August 1995). "Efficacy and tolerability of Casodex in patients with advanced prostate cancer. International Casodex Study Group". Anticancer Drugs 6 (4): 508–13. doi:10.1097/00001813-199508000-00003. PMID 7579554.
- ^ McLeod DG (1997). "Tolerability of Nonsteroidal Antiandrogens in the Treatment of Advanced Prostate Cancer". Oncologist 2 (1): 18–27. PMID 10388026. http://theoncologist.alphamedpress.org/cgi/pmidlookup?view=long&pmid=10388026.
- ^ Mason M (August 2006). "What implications do the tolerability profiles of antiandrogens and other commonly used prostate cancer treatments have on patient care?". J. Cancer Res. Clin. Oncol. 132 Suppl 1: S27–35. doi:10.1007/s00432-006-0134-4. PMID 16896883.
- ^ Eri LM, Haug E, Tveter KJ (March 1995). "Effects on the endocrine system of long-term treatment with the non-steroidal anti-androgen Casodex in patients with benign prostatic hyperplasia". Br J Urol 75 (3): 335–40. doi:10.1111/j.1464-410X.1995.tb07345.x. PMID 7537602.
- ^ Sieber PR (December 2007). "Treatment of bicalutamide-induced breast events". Expert Rev Anticancer Ther 7 (12): 1773–9. doi:10.1586/14737140.7.12.1773. PMID 18062751.
- ^ Kasperk CH, Wergedal JE, Farley JR, Linkhart TA, Turner RT, Baylink DJ (March 1989). "Androgens directly stimulate proliferation of bone cells in vitro". Endocrinology 124 (3): 1576–8. doi:10.1210/endo-124-3-1576. PMID 2521824.
- ^ Manolagas SC, Jilka RL, Girasole G, Passeri G, Bellido T (1993). "Estrogen, cytokines, and the control of osteoclast formation and bone resorption in vitro and in vivo". Osteoporos Int 3 Suppl 1: 114–6. doi:10.1007/BF01621882. PMID 8461536.
- ^ Vanderschueren D, Gaytant J, Boonen S, Venken K (June 2008). "Androgens and bone". Curr Opin Endocrinol Diabetes Obes 15 (3): 250–4. doi:10.1097/MED.0b013e3282fe6ca9. PMID 18438173.
External links
- Casodex, Cosudex (manufacturer's website)
- Bicalutamide (patient information)
Androgens (G03B) Agonist Testosterone# • Androstanolone • Fluoxymesterone • Mesterolone • Methyltestosterone • see also Anabolic steroidsSARM AC-262,356§ • Andarine (S-4)§ • BMS-564,929§ • LGD-2226§ • LGD-3303§ • Ostarine (MK-2866)§ • S-23§ • S-40503§Antiandrogen Bicalutamide • Cyproterone • Dienogest • Flutamide • Galeterone§ • MDV3100† • Nilutamide • SpironolactoneEnzyme inhibitors Alfatradiol • Bexlosteride • Dutasteride • Epristeride • Finasteride • Izonsteride • Lapisteride • TurosterideCYP17A1 inhibitorsCategories:- Antiandrogens
- Nitriles
- Organofluorides
- Anilides
- Alcohols
- Sulfones
Wikimedia Foundation. 2010.
