- Dienogest
-
Dienogest 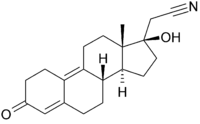
Systematic (IUPAC) name 17-hydroxy-3-oxo-19-nor-17α-pregna-4,9-diene-21-nitrile Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ? Routes Oral Pharmacokinetic data Bioavailability 90%[1] Protein binding 90%[2] Metabolism Hepatic[3] Half-life 6-12 hours[4] Excretion Renal Identifiers CAS number 65928-58-7 
ATC code G03FA15
(combination with estrogen)PubChem CID 68861 UNII 46M3EV8HHE 
KEGG D03799 
ChEMBL CHEMBL1201864 
Chemical data Formula C20H25NO2 Mol. mass 311.42 g/mol[1] Physical data Density 1.2 g/cm³ Boiling point 549 °C (1020 °F)  (what is this?) (verify)
(what is this?) (verify)Dienogest is an orally active synthetic progesterone (or progestin).[5] It is available for use as an oral contraceptive in combination with ethinylestradiol. It has antiandrogenic activity and as a result can improve androgenic symptoms.[1] It is a non-ethinylated progestin which is structurally related to testosterone.[3] Dienogest given in isolation is available for the treatment of endometriosis under the trade name Visanne.
Contents
History
Dienogest was synthesised in 1979 in Jena, Germany under the leadership of Prof. Kurt Ponsold, was initially referred to as STS 557.[6][7] It was found that its potency was 10 times that of levonorgestrel.[8] The first product on the market to contain dienogest as a contraceptive pill Valette in 1995 made by Jenapharm.[9] It has been little used outside of Germany.[10]
Indications
Contraception
Dienogest is used primarily as a contraceptive in combination with ethinylestradiol. It is given as a tablet containing 2 mg of dienogest and 30 μg of ethinylestradiol.[11] The minimum dose required to inhibit ovulation has been found to be approximately 1 mg[12]
Endometriosis
Dienogest is also approved in the European Union for the treatment of endometriosis.[13][14] It has been shown to be equally effective as leuprorelin,[15] which is a second line medication against endometriosis.
Pharmacodynamics
Progestational activity
Dienogest has moderate affinity for the progesterone receptor in human uterus tissue, in vitro, about 10% that of progesterone.[16]
Inhibition of ovulation
The minimum effective dose of oral dienogest required to inhibit ovulation is 1 mg/day.[17] The inhibition of ovulation by dienogest occurs mainly via peripheral action as opposed to central action on gonadotrophin secretion.[1] Oral treatment of dienogest 2 mg/day in cyclical women reduced serum progesterone levels to anovulatory levels, however serum levels of lutenising hormone and follicle-stimulating hormone are not significantly altered.[17]
Adverse effects
Adverse effects associated with dienogest are the same as those expected of a progestogen.[1] These include weight gain, increased blood pressure, breast tenderness and nausea.[18] It produces no androgenic side effects and has little effect on metabolic and lipid haemostatic parameters.[19]
References
- ^ a b c d e Foster RH, Wilde MI (1998). "Dienogest". Drugs 56 (5): 825–33; discussion 834–5. doi:10.2165/00003495-199856050-00007. PMID 9829156.
- ^ de Lignieres B, Dennerstein L, Backstrom T (1995). = MImg&_imagekey = B6T9F-3YCM173-D-1&_cdi = 5113&_user = 308069&_orig = search&_coverDate = 04%2F30%2F1995&_sk = 999789996&view = c&wchp = dGLbVtb-zSkzV&md5 = 661a69b659e2a9afde59f153d419f848&ie = /sdarticle.pdf "Influence of route of administration on progesterone metabolism". Maturitas 21 (3): 251–7. doi:10.1016/0378-5122(94)00882-8. PMID 7616875. http://www.sciencedirect.com/science?_ob = MImg&_imagekey = B6T9F-3YCM173-D-1&_cdi = 5113&_user = 308069&_orig = search&_coverDate = 04%2F30%2F1995&_sk = 999789996&view = c&wchp = dGLbVtb-zSkzV&md5 = 661a69b659e2a9afde59f153d419f848&ie = /sdarticle.pdf.
- ^ a b Stanczyk FZ (2003). = MImg&_imagekey = B6TC9-49W20RK-1-1H&_cdi = 5165&_user = 308069&_orig = search&_coverDate = 11%2F30%2F2003&_sk = 999319989&view = c&wchp = dGLbVtz-zSkWW&md5 = 7d8739b4182a2f943f6463f2b434653d&ie = /sdarticle.pdf "All progestins are not created equal". Steroids 68 (10–13): 879–90. doi:10.1016/j.steroids.2003.08.003. PMID 14667980. http://www.sciencedirect.com/science?_ob = MImg&_imagekey = B6TC9-49W20RK-1-1H&_cdi = 5165&_user = 308069&_orig = search&_coverDate = 11%2F30%2F2003&_sk = 999319989&view = c&wchp = dGLbVtz-zSkWW&md5 = 7d8739b4182a2f943f6463f2b434653d&ie = /sdarticle.pdf.
- ^ Sitruk-Ware R (2004). = MImg&_imagekey = B6T9F-4BY3WYN-7-8&_cdi = 5113&_user = 308069&_orig = search&_coverDate = 04%2F15%2F2004&_sk = 999529995&view = c&wchp = dGLzVzz-zSkzk&md5 = 8fcb633206527789f56a3c28d3956c06&ie = /sdarticle.pdf "Pharmacological profile of progestins". Maturitas 47 (4): 277–83. doi:10.1016/j.maturitas.2004.01.001. PMID 15063480. http://www.sciencedirect.com/science?_ob = MImg&_imagekey = B6T9F-4BY3WYN-7-8&_cdi = 5113&_user = 308069&_orig = search&_coverDate = 04%2F15%2F2004&_sk = 999529995&view = c&wchp = dGLzVzz-zSkzk&md5 = 8fcb633206527789f56a3c28d3956c06&ie = /sdarticle.pdf.
- ^ Nakamura M, Katsuki Y, Shibutani Y, Oikawa T (1999). "Dienogest, a synthetic steroid, suppresses both embryonic and tumor-cell-induced angiogenesis". European Journal of Pharmacology 386 (1): 33–40. doi:10.1016/S0014-2999(99)00765-7. PMID 10611461. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T1J-3Y6GTW1-5-F&_cdi=4892&_user=308069&_orig=search&_coverDate=12%2F10%2F1999&_sk=996139998&view=c&wchp=dGLbVtz-zSkzV&md5=d3bd9b067f0732c4087f0ed6f78ef7cb&ie=/sdarticle.pdf.
- ^ Menzenbach B, Hübner M, K. Ponsold (1984). "Untersuchungen zur Bromierung/Dehydrobromierung von 17-Cyanmethyl-17-hydroxy-östr-5(10)-en-3-on". Journal für Praktische Chemie 326 (6): 893–898. doi:10.1002/prac.19843260606.
- ^ Kaufmann G, Dautzenberg H, Henkel H, et al. (August 1999). "Nitrile hydratase from Rhodococcus erythropolis: metabolization of steroidal compounds with a nitrile group". Steroids 64 (8): 535–40. doi:10.1016/S0039-128X(99)00028-8. PMID 10493599. http://linkinghub.elsevier.com/retrieve/pii/S0039-128X(99)00028-8.
- ^ Oettel M, Kurischko A (1980). "STS 557, a new orally active progestin with antiprogestational and contragestational properties in rabbits". Contraception 21 (1): 61–9. doi:10.1016/0010-7824(80)90140-7. PMID 7357870. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T5P-4BWV44D-7-1&_cdi=5008&_user=308069&_orig=search&_coverDate=01%2F31%2F1980&_sk=999789998&view=c&wchp=dGLbVtb-zSkzk&md5=ce10dd4f44f22ea913e52651be02fe5b&ie=/sdarticle.pdf.
- ^ http://www.jenapharm.de/unternehmen/ueber-uns/geschichte/1965-1995/
- ^ Kuhl H (1998). "Dienogest. A Viewpoint by Herbert Kuhl". Drugs 56 (5): 834.
- ^ Wiegratz I, Mittmann K, Dietrich H, Zimmermann T, Kuhl H (2006). "Fertility after discontinuation of treatment with an oral contraceptive containing 30 microg of ethinyl estradiol and 2 mg of dienogest". Fertil. Steril. 85 (6): 1812–9. doi:10.1016/j.fertnstert.2005.11.052. PMID 16759929. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T6K-4K48058-D-7&_cdi=5033&_user=308069&_orig=search&_coverDate=06%2F30%2F2006&_sk=999149993&view=c&wchp=dGLbVtz-zSkWA&md5=09a9c18943af939b02ba9d76b2edb79b&ie=/sdarticle.pdf.
- ^ Moore C, Carol W, Gräser T, Mellinger U, Walter F (1999). "Influence of Dienogest on Ovulation in Young Fertile Women". Clinical Drug Investigation 18 (4): 271–278. doi:10.1016/j.fertnstert.2005.11.052. PMID 16759929. http://www.medscape.com/viewarticle/406143.
- ^ "Dienogest for the treatment of endometriosis" (PDF). London New Drugs Group. http://www.nelm.nhs.uk/en/Download/?file=MDs3NTMyMTA7L3VwbG9hZC9EaWVub2dlc3RfU2VwdF8xMC5wZGY_.pdf. Retrieved 2010-12-07.
- ^ "Visanne" (in German). Netdoctor.de. http://www.netdoktor.de/Medikamente/Visanne-r-100012124.html.
- ^ Strowitzki, T; Marr, J; Gerlinger, C; Faustmann, T; Seitz, C (2010). "Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial". Human reproduction (Oxford, England) 25 (3): 633–41. doi:10.1093/humrep/dep469. PMID 20089522.
- ^ Oettel M, Bervoas-Martin S, Elger W, Golbs S, Hobe G, Kaufmann G, Mathieu M, Moore C, Schneider B, Puri C, Ritter P, Reddersen G, Schon R, Strauch G, Zimmermann H (1995). "A 19-norprogestin without 17α-ethinyl group II: Dienogest from a pharmacokinetic point of view". Drugs of Today 31 (7): 499–516.
- ^ a b Oettel M, Carol W, Elger W, Kaufmann G, Moore C, Romer W, Klinger G, Schneider B, Schroder J, Sobek L, Walter F, Zimmermann H (1995). "A 19-norprogestin without 17α-ethinyl group II: Dienogest from a pharmacodynamic point of view". Drugs of Today 31 (7): 517–536.
- ^ Galbraith, Alan; Shane Bullock, Elizabeth Manias, Barry Hunt, Ann Richards (2007). Fundamentals of Pharmacology: An Applied Approach for Nursing and Health. United Kingdom: Pearson Education LTD. pp. 632. ISBN 978-0131869011. http://www.pearsoned.co.uk/Bookshop/detail.asp?item=100000000107920.
- ^ Wiegratz I, Lee JH, Kutschera E, Bauer HH, von Hayn C, Moore C, Mellinger U, Winkler UH, Gross W, Kuhl H (2002). "Effect of dienogest-containing oral contraceptives on lipid metabolism". Contraception 65 (3): 223–9. doi:10.1016/S0010-7824(01)00310-9. PMID 11929644. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T5P-45FD507-5-9&_cdi=5008&_user=308069&_orig=search&_coverDate=03%2F31%2F2002&_sk=999349996&view=c&wchp=dGLbVzW-zSkWb&md5=6a7fa2b0616afba9866f9c0dd1b11b48&ie=/sdarticle.pdf.
Androgens (G03B) Agonist Testosterone# • Androstanolone • Fluoxymesterone • Mesterolone • Methyltestosterone • see also Anabolic steroidsSARM AC-262,356§ • Andarine (S-4)§ • BMS-564,929§ • LGD-2226§ • LGD-3303§ • Ostarine (MK-2866)§ • S-23§ • S-40503§Antiandrogen Bicalutamide • Cyproterone • Dienogest • Flutamide • Galeterone§ • MDV3100† • Nilutamide • SpironolactoneEnzyme inhibitors Alfatradiol • Bexlosteride • Dutasteride • Epristeride • Finasteride • Izonsteride • Lapisteride • TurosterideCYP17A1 inhibitorsAbiraterone • Galeterone§ • KetoconazoleEstrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneAsoprisnil • CDB-4124 • Ulipristal acetateEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • Toremifenepure antagonist: FulvestrantCategories:- Antiandrogens
- Nitriles
- Alcohols
- Ketones
Wikimedia Foundation. 2010.
