- Clomifene
-
Clomifene 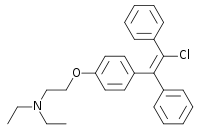
Systematic (IUPAC) name 2-(4-(2-chloro-1,2-diphenylethenyl)
phenoxy)-N,N-diethyl-ethanamineClinical data AHFS/Drugs.com Micromedex Detailed Consumer Information Pregnancy cat. B3 (Au), X (U.S.) Legal status S4 (Au), POM (UK), ℞-only (U.S.) Routes 50 mg tablets Pharmacokinetic data Bioavailability High (>90%) Metabolism Hepatic (with enterohepatic circulation) Half-life 5-7 days Excretion Mainly renal, some biliary Identifiers CAS number 911-45-5 
ATC code G03GB02 PubChem CID 2800 DrugBank APRD00880 ChemSpider 2698 
UNII 1HRS458QU2 
KEGG D07726 
ChEBI CHEBI:3752 
ChEMBL CHEMBL167779 
Chemical data Formula C26H28ClNO Mol. mass 406 or 598.10 (with citrate) SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Clomifene (INN) or clomiphene (USAN and former BAN) or Clomid or Clomifert is a selective estrogen receptor modulator (SERM) that increases production of gonadotropins by inhibiting negative feedback on the hypothalamus. It is used mainly in female infertility, in turn mainly as ovarian stimulation to reverse oligoovulation or anovulation such as in infertility in polycystic ovary syndrome, as well as being used for ovarian hyperstimulation, such as part of an in vitro fertilization procedure. Clomifene citrate is marketed under various trade names including Clomid, Serophene, and Milophene.
Contents
Mode of action
Clomifene appears to inhibit estrogen receptors in hypothalamus, thereby inhibiting negative feedback of estrogen on gonadotropin production.[1] It may also result in direct stimulation of the hypothalamic-pituitary axis.[1] Zuclomifene, a more active isomer, stays bound for long periods of time.
In normal physiologic female hormonal cycling, at 7 days past ovulation, high levels of estrogen and progesterone produced from the corpus luteum inhibit GnRH, FSH and LH at the hypothalamus and anterior pituitary. If fertilization does not occur in the post-ovulation period the corpus luteum disintegrates due to a lack of beta-hCG. This would normally be produced by the embryo in the effort of maintaining progesterone and estrogen levels during pregnancy.
Therapeutically, clomifene is given early in the menstrual cycle. It is typically prescribed beginning on day 1, 3 or 5 and continuing for 5 days. By that time, FSH level is rising steadily, causing development of a few follicles. Follicles in turn produce the estrogen, which circulates in serum. In the presence of clomifene, the body perceives a low level of estrogen, similar to day 22 in the previous cycle. Since estrogen can no longer effectively exert negative feedback on the hypothalamus, GnRH secretion becomes more rapidly pulsatile, which results in increased pituitary gonadotropin (FSH, LH) release. (It should be noted that more rapid, lower amplitude pulses of GnRH lead to increased LH/FSH secretion, while more irregular, larger amplitude pulses of GnRH leads to a decrease in the production of LH/FSH.) Increased FSH level causes growth of more ovarian follicles, and subsequently rupture of follicles resulting in ovulation.
Chemistry
Clomifene is a mixture of two geometric isomers, enclomifene (E-clomifene) and zuclomifene (Z-clomifene).
 Enclomifene
Enclomifene Zuclomifene
ZuclomifeneAdverse effects
Common adverse drug reactions associated with the use of clomifene (≥1% of patients) include: vasomotor flushes (or hot flashes ), abdominal discomfort, visual blurring (dose-dependent), and/or reversible ovarian enlargement and cyst formation. Infrequent adverse effects (0.1–1% of patients) include: abnormal uterine bleeding, nausea, and/or vomiting. Rare adverse effects (<0.1% of patients) include: reversible alopecia and/or ovarian hyperstimulation syndrome.[2]
Clomifene can lead to multiple ovulation, hence increasing the chance of twins (10% of births instead of the normal ~1%). In comparison to treatment with purified FSH, the rate of ovarian hyperstimulation syndrome is low.
Off-label use in the treatment of male hypogonadism (Low T)
Clomifene citrate has been found very effective in the treatment of secondary male hypogonadism in many cases.[3] This has shown to be a much more attractive option than testosterone replacement therapy (TRT) in many cases because of the reduced cost and convenience of taking a pill as opposed to testosterone injections or gels.[4] Unlike traditional TRT it also does not shrink the testes and as a result can enhance fertility. Traditional TRT can render a man sterile (although with careful monitoring and low-dose HCG as an adjunct, this is both preventable and reversible for most men).[5] Because clomifene citrate has not been FDA approved for use in males it is prescribed off-label. According to Professor Craig Niederberger, because this drug is now generic, no drug company would pursue FDA approval for use in men now because of limited profit incentive, mostly due to the relatively small market potential.[6] However, the single isomer of clomifene "enclomiphene" under the brand name Androxal is currently under phase 2 trials for use in men.[7][8]
Prohibited use in sports and use in bodybuilding
Clomifene is commonly used by male anabolic steroid users to bind the estrogen receptors in their bodies, thereby blocking the effects of estrogen, such as gynecomastia. It also restores the body's natural production of testosterone. It is commonly used as a "recovery drug" and taken toward the end of a steroid cycle.[citation needed] It is included on the World Anti-Doping Agency list of illegal doping agents in sport.[9]
History
Clomifene has been used since the 1960s.[10] It was first used to treat cases of oligomenorrhea, and it was then applied to the treatment of anovulation.[11]
References
- ^ a b DrugBank > Clomifene. Updated on April 19, 2011
- ^ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3[page needed]
- ^ Ioannidoukadis, Stella; Wright, Pat J.; Neely, R. Dermot; Quinton, Richard (2006). "Complete reversal of adult-onset isolated hypogonadotropic hypogonadism with clomiphene citrate". Fertility and Sterility 86 (5): 1513.e5–9. doi:10.1016/j.fertnstert.2006.03.065. PMID 17070201.
- ^ Taylor, Frederick; Levine, Laurence (2010). "Clomiphene Citrate and Testosterone Gel Replacement Therapy for Male Hypogonadism: Efficacy and Treatment Cost". Journal of Sexual Medicine 7 (1 Pt 1): 269–76. doi:10.1111/j.1743-6109.2009.01454.x. PMID 19694928.
- ^ Kaminetsky, Jed; Hemani, Micah L (2009). "Clomiphene citrate and enclomiphene for the treatment of hypogonadal androgen deficiency". Expert Opinion on Investigational Drugs 18 (12): 1947–55. doi:10.1517/13543780903405608. PMID 19938905.
- ^ How Clomid Works in Men
- ^ Androxal website
- ^ Hill, S.; Arutchelva, V.; Quinton, R. (2009). "Enclomiphene, an estrogen receptor antagonist for the treatment of testosterone deficiency in men". IDrugs 12 (2): 109–19. PMID 19204885.
- ^ The WADA Prohibited List
- ^ Holtkamp DE, Greslin JG, Root CA, Lerner LJ (October 1960). "Gonadotrophin inhibiting and anti-fecundity effects of chloramiphene". Proc. Soc. Exp. Biol. Med. 105: 197–201. PMID 13715563.
- ^ Hughes E, Collins J, Vandekerckhove P (2000). "Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea". Cochrane Database Syst Rev (2): CD000056. doi:10.1002/14651858.CD000056. PMID 10796477.
External links
Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • ToremifeneCategories:- Citrates
- Selective estrogen receptor modulators
- Stilbenoid drugs
- Phenol ethers
- Organochlorides
Wikimedia Foundation. 2010.
