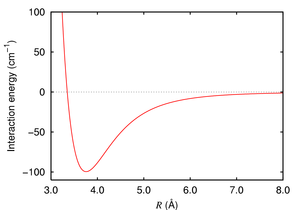
- London dispersion force
-
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces) is a type of force acting between atoms and molecules.[1] They are part of the van der Waals forces. The LDF is named after the German-American physicist Fritz London.
The LDF is a weak intermolecular force arising from quantum induced instantaneous polarization multipoles in molecules. They can therefore act between molecules without permanent multipole moments.
London forces are exhibited by nonpolar molecules because of the correlated movements of the electrons in interacting molecules. Because the electrons from different molecules start "feeling" and avoiding each other, electron density in a molecule becomes redistributed in proximity to another molecule, (see quantum mechanical theory of dispersion forces). This is frequently described as formation of "instantaneous dipoles" that attract each other. London forces are present between all chemical groups and usually represent the main part of the total interaction force in condensed matter, even though they are generally weaker than ionic bonds and hydrogen bonds.
This is the only attractive intermolecular force present between neutral atoms (e.g., a noble gas). Without London forces, there would be no attractive force between noble gas atoms, and they wouldn't exist in liquid form.
London forces become stronger as the atom or molecule in question becomes larger. This is due to the increased polarizability of molecules with larger, more dispersed electron clouds. This trend is exemplified by the halogens (from smallest to largest: F2, Cl2, Br2, I2). Fluorine and chlorine are gases at room temperature, bromine is a liquid, and iodine is a solid. The London forces also become stronger with larger amounts of surface contact. Greater surface area means closer interaction between different molecules.
Quantum mechanical theory of dispersion forces
The first explanation of the attraction between noble gas atoms was given by Fritz London in 1930.[2][3][4] He used a quantum mechanical theory based on second-order perturbation theory. The perturbation is the Coulomb interaction V between the electrons and nuclei of the two monomers (atoms or molecules) that constitute the dimer. The second-order perturbation expression of the interaction energy contains a sum over states. The states appearing in this sum are simple products of the stimulated electronic states of the monomers. Thus, no intermolecular antisymmetrization of the electronic states is included and the Pauli exclusion principle is only partially satisfied.
London developed the method perturbation V in a Taylor series in
 , where R is the distance between the nuclear centers of mass of the monomers.
, where R is the distance between the nuclear centers of mass of the monomers.This Taylor expansion is known as the multipole expansion of V because the terms in this series can be regarded as energies of two interacting multipoles, one on each monomer. Substitution of the multipole-expanded form of V into the second-order energy yields an expression that resembles somewhat an expression describing the interaction between instantaneous multipoles (see the qualitative description above). Additionally an approximation, named after Albrecht Unsöld, must be introduced in order to obtain a description of London dispersion in terms of dipole polarizabilities and ionization potentials.
In this manner the following approximation is obtained for the dispersion interaction
 between two atoms A and B. Here αA and αB are the dipole polarizabilities of the respective atoms. The quantities IA and IB are the first ionization potentials of the atoms and R is the intermolecular distance.
between two atoms A and B. Here αA and αB are the dipole polarizabilities of the respective atoms. The quantities IA and IB are the first ionization potentials of the atoms and R is the intermolecular distance.
Note that this final London equation does not contain instantaneous dipoles (see molecular dipoles). The "explanation" of the dispersion force as the interaction between two such dipoles was invented after London gave the proper quantum mechanical theory. See the authoritative work[5] for a criticism of the instantaneous dipole model and[6] for a modern and thorough exposition of the theory of intermolecular forces.
The London theory has much similarity to the quantum mechanical theory of light dispersion, which is why London coined the phrase "dispersion effect".
Relative magnitude
Dispersion forces are usually dominant of the three van der Waals forces (orientation, induction, dispersion) between atoms and molecules, with the exception for molecules that are small and highly polar, like of water. The following contribution of the dispersion to the total intermolecular interaction energy has been given:[7]
Contribution of the dispersion to the total intermolecular interaction energy Molecule pair % of the total energy of interaction Ne-Ne 100 CH4-CH4 100 HCl-HCl 86 HBr-HBr 96 HI-HI 99 CH3Cl-CH3Cl 68 NH3-NH3 57 H2O-H2O 24 HCl-HI 96 H2O-CH4 87 References
- ^ http://www.youtube.com/watch?v=UWHUUBsNFbY
- ^ R. Eisenschitz and F. London, Z. Physik 60, 491 (1930)
- ^ F. London, Z. Physik 63, 245 (1930) and Z. Physik. Chemie, 33, 8-26 (1937). English translations in H. Hettema, Quantum Chemistry, Classic Scientific Papers, World Scientific, Singapore (2000).
- ^ F. London, Transactions of the Faraday Society 33, 8-26 (1937)
- ^ J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, Wiley, New York, 1954
- ^ A. J. Stone, The Theory of Intermolecular Forces, 1996, (Clarendon Press, Oxford)
- ^ J. Israelachvili, "Intermolecular and Surface Forces", 2nd edition, Academic Press, 1992.
Chemical bonds Intramolecular
("strong")Sigma bond · Pi bond · Delta bond
Double bond · Triple bond · Quadruple bond · Quintuple bond · Sextuple bond
3c–2e · 3c–4e · 4c–2e
Agostic bond · Bent bond · Dipolar bond · Pi backbond
Conjugation · Hyperconjugation · Aromaticity · Hapticity · AntibondingCation–pi bond · Salt bondIntermolecular
("weak")Other noncovalentvan der Waals force · London dispersion force · Mechanical bond · Halogen bond · Aurophilicity · Intercalation · Stacking · Entropic force · Chemical polarityNote: the weakest strong bonds are not necessarily stronger than the strongest weak bonds Categories:- Chemical bonding
- Intermolecular forces
Wikimedia Foundation. 2010.