- Cumene hydroperoxide
-
Cumene hydroperoxide[1] 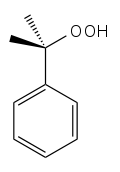 2-hydroperoxypropan-2-ylbenzeneOther namesCumyl Hydroperoxide
2-hydroperoxypropan-2-ylbenzeneOther namesCumyl Hydroperoxide
CHPIdentifiers CAS number 80-15-9 
PubChem 6629 ChemSpider 6377 
Jmol-3D images Image 1 - OOC(c1ccccc1)(C)C
Properties Molecular formula C9H12O2 Molar mass 152.19 g mol−1 Appearance colorless to pale yellow liquid Density 1.02 g/cm3 Melting point -9 °C, 264 K, 16 °F
Boiling point ca. 125 °C (decomposes, possibly explosively)
Solubility in water 1.5 g / 100 mL Vapor pressure 14 mmHg at 20 °C Hazards NFPA 704  hydroperoxide (verify) (what is:
hydroperoxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cumene hydroperoxide is an intermediate in the cumene process for developing phenol and acetone from benzene and propylene. It is typically used as an oxidising agent.[2] Products of decomposition of cumene hydroperoxide are methylstyrene, acetophenone and cumyl alcohol.[3] Its formula is C6H5C(CH3)2OOH.
References
- ^ University, Safety Officer in Physical Chemistry at Oxford (2005). "Safety (MSDS) data for cumene hydroperoxide". http://msds.chem.ox.ac.uk/CU/cumene_hydroperoxide.html. Retrieved 2009-05-13
- ^ Richard J. Lewis, Richard J. Lewis (Sr.), Hazardous chemicals desk reference, Publisher Wiley-Interscience, 2008, ISBN 0470180242, 9780470180242, 1953 pages (page 799)
- ^ Cumene Hydroperoxide at the Organic Chemistry Portal
Related terms
External links
- Cumene hydroperoxide
- Cumene hydroperoxide at International Chemical Safety Cards
Categories:- Organic peroxides
Wikimedia Foundation. 2010.

