- Bis(benzene)chromium
-
Bis(benzene)chromium 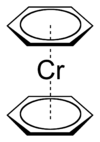
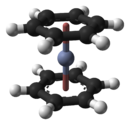
 Bis(benzene) chromiumOther namesdi(benzene) chromium
Bis(benzene) chromiumOther namesdi(benzene) chromium
dibenzenechromiumIdentifiers CAS number 1271-54-1 RTECS number GB5850000 Properties Molecular formula C12H12Cr Molar mass 208.22 g/mol Appearance brown-black crystals Melting point 284-285 °C
Boiling point sublimes:160 °C
in vacuo.Solubility in water insoluble Solubility in other solvents slightly: benzene, THF Structure Coordination
geometrypseudooctahedral Dipole moment 0 D Hazards R-phrases 11 S-phrases 16-33 Main hazards flammable Flash point 180 °F Related compounds Related compounds Ferrocene
chromocene (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Bis(benzene)chromium is the organometallic compound with the formula Cr(η6-C6H6)2. It is sometimes called dibenzenechromium. The compound played an important role in the development of sandwich compounds in organometallic chemistry and is the prototypical complex containg two arene ligands.
Preparation
The substance is air sensitive and its synthesis requires air-free techniques. It was first prepared by Hafner and Fischer by the reaction of CrCl3, aluminium, and benzene, in the presence of AlCl3. This so-called reductive Friedel-Crafts method was pioneered by E.O. Fischer and his students.[1][2] The product of the reaction was yellow [Cr(C6H6)2]+, which was then reduced to the neutral complex. Idealized equations for the synthesis are:
- CrCl3 + 2/3 Al + 1/3 AlCl3 + 2 C6H6 → [Cr(C6H6)2]AlCl4 + 2/3 AlCl3
- [Cr(C6H6)2]AlCl4 + 1/2 Na2S2O4 → [Cr(C6H6)2] + NaAlCl4 + SO2
Compounds closely related to [Cr(C6H6)2]+ had been prepared many years before Fischer's work by Franz Hein by the reaction of phenylmagnesium bromide and CrCl3.[3] Hein's reaction affords cationic sandwich complexes containing bi- and terphenyl, which baffled chemists until the breakthrough by Fischer and Hafner,[4] and thus Hein had unknowingly discovered sandwich complexes a half century ahead of the work on ferrocene. Fischer and Seus soon prepared Hein's [Cr(C6H5-C6H5)2]+.[5][6] Illustrating the rapid pace of this research, the same issue of Chem. Ber. also describes the Mo(0) complex.[7]
Reactions
The compound reacts with carboxylic acids to give chromium(II) carboxylates, such as chromium(II) acetate, which have interesting structures. Oxidation affords [Cr(C6H6)2]+. Carbonylation gives (benzene)chromium tricarbonyl.
The compound finds limited use in organic synthesis.[8]
References
- ^ King, R. B. Organometallic Syntheses. Volume 1 Transition-Metal Compounds; Academic Press: New York, 1965. ISBN 0-444-42607-8
- ^ Elschenbroich, C.; Salzer, A. ”Organometallics : A Concise Introduction” (2nd Ed) (1992) Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- ^ Seyferth, D. (2002). "Bis(benzene)chromium. 1. Franz Hein at the University of Leipzig and Harold Zeiss and Minoru Tsutsui at Yale". Organometallics 21 (8): 1520–1530. doi:10.1021/om0201056.
- ^ Seyferth, D. (2002). "Bis(benzene)chromium. 2. Its Discovery by E. O. Fischer and W. Hafner and Subsequent Work by the Research Groups of E. O. Fischer, H. H. Zeiss, F. Hein, C. Elschenbroich, and Others". Organometallics 21 (14): 2800–2820. doi:10.1021/om020362a.
- ^ Fischer, E. O; Seus, D. (1956). "Zur Frage der Struktur der Chrom-phenyl-Verbindungen. Über Aromatenkomplexe von Metallen VI". Chemische Berichte 89 (8): 1809–1815. doi:10.1002/cber.19560890803.
- ^ Hein, F. (1956). "Zur Frage der Struktur der Chrom-phenyl-Verbindungen. Bemerkungen zur Abhandlung von E. O. Fischer und D. Seus". Chemische Berichte 89 (8): 1816–1821. doi:10.1002/cber.19560890804.
- ^ Fischer, E. O.; Stahl, H.-O. (1956,). "Di-benzol-molybdän (O). Über Aromatenkomplexe von Metallen V". Chemische Berichte 89 (8): 1805–1808. doi:10.1002/cber.19560890802.
- ^ Herndon, J. W. "Dibenzenechromium" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. DOI: 10.1002/047084289.
Categories:- Organochromium compounds
- Sandwich compounds
Wikimedia Foundation. 2010.
