- Oxolinic acid
-
Oxolinic acid 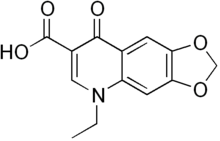
Systematic (IUPAC) name 5-Ethyl- 8-oxo- 5,8-dihydro [1,3] dioxolo [4,5-g] quinoline- 7-carboxylic acid Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ? Identifiers CAS number 14698-29-4 
ATC code J01MB05 PubChem CID 4628 UNII L0A22B22FT 
KEGG D02301 
ChEMBL CHEMBL416755 
Chemical data Formula C13H11NO5 Mol. mass 261.23 g/mol  (what is this?) acid (verify)
(what is this?) acid (verify)Oxolinic acid is a quinolone antibiotic developed in Japan in the 1970s[1][2]. Dosages 12-20mg/kg orally administered for five to ten days. The antibiotic works by inhibiting the enzyme DNA gyrase. It also acts as a dopamine reuptake inhibitor and has stimulant effects in mice.[3]
See also
- Amfonelic acid
- Fluoroquinolone
References
- ^ JP Patent 49138244
- ^ Gleckman R, Alvarez S, Joubert DW, Matthews SJ (August 1979). "Drug therapy reviews: oxolinic acid". American Journal of Hospital Pharmacy 36 (8): 1077–9. PMID 384788.
- ^ Garcia de Mateos-Verchere J, Vaugeois JM, Naudin B, Costentin J (December 1998). "Behavioural and neurochemical evidence that the antimicrobial agent oxolinic acid is a dopamine uptake inhibitor". European Neuropsychopharmacology : the Journal of the European College of Neuropsychopharmacology 8 (4): 255–9. doi:10.1016/S0924-977X(97)00083-7. PMID 9928913. http://linkinghub.elsevier.com/retrieve/pii/S0924-977X(97)00083-7.
Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Sulfonamides
(DHPS inhibitor)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.Cinoxacin‡ • Flumequine • Nalidixic acid • Oxolinic acid • Pipemidic acid • Piromidic acid • Rosoxacin2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis 
This systemic antibacterial-related article is a stub. You can help Wikipedia by expanding it.
