- Dihydrofolate reductase
-
Dihydrofolate reductase 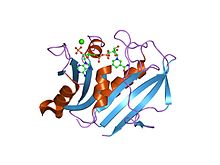
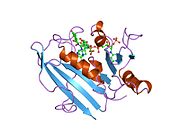
refined crystal structures of chicken liver dihydrofolate reductase. 3 angstroms apo-enzyme and 1.7 angstroms nadph holo-enzyme complex Identifiers Symbol DHFR_1 Pfam PF00186 Pfam clan CL0387 InterPro IPR001796 PROSITE PDOC00072 SCOP 1dhi Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary R67 dihydrofolate reductase 
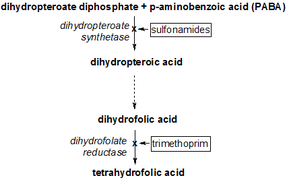
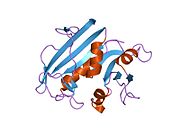
high-resolution structure of a plasmid-encoded dihydrofolate reductase: pentagonal network of water molecules in the d2-symmetric active site Identifiers Symbol DHFR_2 Pfam PF06442 InterPro IPR009159 SCOP 1vif Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the DHFR gene.[1][2] It is found in the q11→q22 region of chromosome 5.[3]
Bacterial species possesses distinct DHFR enzymes (based on their pattern of binding diaminoheterocyclic molecules), but mammalian DHFRs are highly similar.[4] The plasmid-encoded DHFR (R67 dihydrofolate reductase) shows a high level of resistance to the antibiotic trimethoprim. It is a homotetramer with an unusual pore, which contains the active site, passing through the middle of the molecule. Its structure is unrelated to that of chromosomal DHFRs.[5]
Contents
Structure
A central eight-stranded beta-pleated sheet makes up the main feature of the polypeptide backbone folding of DHFR.[6] Seven of these strands are parallel and the eighth runs antiparallel. Four alpha helices connect successive beta strands.[7] Residues 9 – 24 are termed “Met20” or “loop 1” and, along with other loops, are part of the major subdomain that surround the active site.[8] The active site is situated in the N-terminal half of the sequence, which includes a conserved Pro-Trp dipeptide; the tryptophan has been shown to be involved in the binding of substrate by the enzyme.[9]
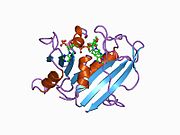
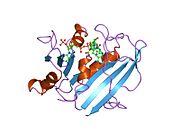
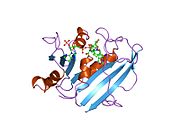
 Human DHFR with bound dihydrofolate and NADPH
Human DHFR with bound dihydrofolate and NADPHFunction
Dihydrofolate reductase converts dihydrofolate into tetrahydrofolate, a methyl group shuttle required for the de novo synthesis of purines, thymidylic acid, and certain amino acids. While the functional dihydrofolate reductase gene has been mapped to chromosome 5, multiple intronless processed pseudogenes or dihydrofolate reductase-like genes have been identified on separate chromosomes.[10]
Reaction catalyzed by DHFR.Tetrahydrofolate synthesis pathway.Mechanism
DHFR catalyzes the transfer of a hydride from NADPH to dihydrofolate with an accompanying protonation to produce tetrahydrofolate.[11] In the end, dihydrofolate is reduced to tetrahydrofolate and NADPH is oxidized to NADP+. The high flexibility of Met20 and other loops near the active site play a role in promoting the release of the product, tetrahydrofolate. In particular the Met20 loop helps stabilize the nicotinamide ring of the NADPH to promote the transfer of the hydride from NADPH to dihydrofolate.[8]
 The reduction of dihydrofolate to tetrahydrofolate.
The reduction of dihydrofolate to tetrahydrofolate.Biological Function
Found in all organisms, DHFR has a critical role in regulating the amount of tetrahydrofolate in the cell. Tetrahydrofolate and its derivatives are essential for purine and thymidylate synthesis, which are important for cell proliferation and cell growth.[11] DHFR plays a central role in the synthesis of nucleic acid precursors, and it has been shown that mutant cells that completely lack DHFR require glycine, a purine, and thymidine to grow.[12]
Clinical significance
Dihydrofolate reductase deficiency has been linked to megaloblastic anemia.[10] Treatment is with reduced forms of folic acid. Because tetrahydrofolate, the product of this reaction, is the active form of folate in humans, inhibition of DHFR can cause functional folate deficiency. Its central role in DNA precursor synthesis, coupled with its inhibition by antagonists such as trimethoprim and methotrexate, which are used as anti-bacterial or anti-cancer agents, has made DHFR a target of anticancer chemotherapy. However, resistance has developed against some drugs, as a result of changes in DHFR itself.[13]
Therapeutic application and Disease Relevance
Since folate is needed by rapidly dividing cells to make thymine, this effect may be used to therapeutic advantage.
DHFR can be target in the treatment of cancer. DHFR is responsible for the levels of tetrahydrofolate in a cell, and the inhibition of DHFR can limit the growth and proliferation of cells that are characteristic of cancer. Methotrexate, a competitive inhibitor of DHFR, is one such anticancer drug that inhibits DHFR.[14] Other drugs include trimethoprim and pyrimethamine. These 3 are widely used as antitumor and antimicrobial agents.[15] Whether or not these are potent anticancer agents is unclear.
Trimethoprim has shown to have activity against a variety of Gram-positive bacterial pathogens.[16] However, resistance to trimethoprim and other drugs aimed at DHFR can arise due to a variety of mechanisms, limiting the success of their therapeutical uses.[17][18] Resistance can arise from DHFR gene amplification, mutations in DHFR, decrease in the uptake of the drugs, among others. Regardless, trimethoprim and sulfamethoxazole in combination has been used as an antibacterial agent for decades.[16]
Folic acid is necessary for growth, and the pathway of the metabolism of folic acid is a target in developing treatments for cancer. DHFR is one such target. A regimen of fluorouracil, doxorubicin, and methotrexate was shown to prolong survival in patients with advanced gastric cancer.[19] Further studies into inhibitors of DHFR can lead to more ways to treat cancer.
Interactions
Dihydrofolate reductase has been shown to interact with GroEL[20] and Mdm2.[21]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective Wikipedia articles. [22]
References
- ^ Chen MJ, Shimada T, Moulton AD, Harrison M, Nienhuis AW (December 1982). "Intronless human dihydrofolate reductase genes are derived from processed RNA molecules". Proc. Natl. Acad. Sci. U.S.A. 79 (23): 7435–9. doi:10.1073/pnas.79.23.7435. PMC 347354. PMID 6961421. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=347354.
- ^ Chen MJ, Shimada T, Moulton AD, Cline A, Humphries RK, Maizel J, Nienhuis AW (March 1984). "The functional human dihydrofolate reductase gene". J. Biol. Chem. 259 (6): 3933–43. PMID 6323448. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=6323448.
- ^ Funanage VL, Myoda TT, Moses PA, Cowell HR (October 1984). "Assignment of the human dihydrofolate reductase gene to the q11----q22 region of chromosome 5". Mol. Cell. Biol. 4 (10): 2010–6. PMC 369017. PMID 6504041. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=369017.
- ^ Smith SL, Patrick P, Stone D, Phillips AW, Burchall JJ (November 1979). "Porcine liver dihydrofolate reductase. Purification, properties, and amino acid sequence". J. Biol. Chem. 254 (22): 11475–84. PMID 500653.
- ^ Narayana N, Matthews DA, Howell EE, Nguyen-huu X (November 1995). "A plasmid-encoded dihydrofolate reductase from trimethoprim-resistant bacteria has a novel D2-symmetric active site". Nat. Struct. Biol. 2 (11): 1018–25. doi:10.1038/nsb1195-1018. PMID 7583655.
- ^ Matthews DA, Alden RA, Bolin JT, Freer ST, Hamlin R, Xuong N, Kraut J, Poe M, Williams M, Hoogsteen K (July 1977). "Dihydrofolate reductase: x-ray structure of the binary complex with methotrexate". Science 197 (4302): 452–455. doi:10.1126/science.17920. PMID 17920.
- ^ Filman DJ, Bolin JT, Matthews DA, Kraut J. (November 1982). "Crystal structure of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 A resolution. II. Environment of bound NADPH and implications for catalysis". The Journal of Biological Chemistry 257 (22): 13650–13662. PMID 6815179.
- ^ a b Osborne MJ, Schnell J, Benkovic SJ, Dyson HJ, Wright PE (August 2001). "Backbone dynamics in dihydrofolate reductase complexes: role of loop flexibility in the catalytic mechanism". Biochemistry 40 (33): 9846–59. doi:10.1021/bi010621k. PMID 11502178.
- ^ Bolin JT, Filman DJ, Matthews DA, Hamlin RC, Kraut J (November 1982). "Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 A resolution. I. General features and binding of methotrexate". J. Biol. Chem. 257 (22): 13650–62. PMID 6815178.
- ^ a b "Entrez Gene: DHFR dihydrofolate reductase". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1719.
- ^ a b Schnell JR, Dyson HJ, Wright PE (June 2004). "Structure, dynamics, and catalytic function of dihydrofolate reductase.". Annual Review of Biophysics and Biomolecular Structure 33 (1): 119–40. doi:10.1146/annurev.biophys.33.110502.133613. PMID 15139807.
- ^ Urlaub G, Chasin LA (July 1980). "Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity". Proc. Natl. Acad. Sci. U.S.A. 77 (7): 4216–20. doi:10.1073/pnas.77.7.4216. PMC 349802. PMID 6933469. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=349802.
- ^ Cowman AF, Lew AM (November 1989). "Antifolate drug selection results in duplication and rearrangement of chromosome 7 in Plasmodium chabaudi". Mol. Cell. Biol. 9 (11): 5182–8. PMC 363670. PMID 2601715. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=363670.
- ^ Li R, Sirawaraporn R, Chitnumsub P, et al. (January 2000). "Three-dimensional structure of M. tuberculosis dihydrofolate reductase reveals opportunities for the design of novel tuberculosis drugs". J. Mol. Biol. 295 (2): 307–23. doi:10.1006/jmbi.1999.3328. PMID 10623528.
- ^ Benkovic SJ, Fierke CA, Naylor AM (March 1988). "Insights into enzyme function from studies on mutants of dihydrofolate reductase". Science 239 (4844): 1105–10. doi:10.1126/science.3125607. PMID 3125607.
- ^ a b Hawser S, Lociuro S, Islam K (March 2006). "Dihydrofolate reductase inhibitors as antibacterial agents". Biochem. Pharmacol. 71 (7): 941–8. doi:10.1016/j.bcp.2005.10.052. PMID 16359642.
- ^ Huennekens FM (June 1996). "In search of dihydrofolate reductase". Protein Sci. 5 (6): 1201–8. doi:10.1002/pro.5560050626. PMC 2143423. PMID 8762155. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2143423.
- ^ Banerjee D, Mayer-Kuckuk P, Capiaux G, Budak-Alpdogan T, Gorlick R, Bertino JR (July 2002). "Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase". Biochim. Biophys. Acta 1587 (2-3): 164–73. doi:10.1016/S0925-4439(02)00079-0. PMID 12084458.
- ^ Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M (July 1993). "Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer". Cancer 72 (1): 37–41. doi:10.1002/1097-0142(19930701)72:1<37::AID-CNCR2820720109>3.0.CO;2-P. PMID 8508427.
- ^ Mayhew, M; da Silva A C, Martin J, Erdjument-Bromage H, Tempst P, Hartl F U (Feb. 1996). "Protein folding in the central cavity of the GroEL-GroES chaperonin complex". Nature (ENGLAND) 379 (6564): 420–6. doi:10.1038/379420a0. ISSN 0028-0836. PMID 8559246.
- ^ Maguire, Maria; Nield Paul C, Devling Timothy, Jenkins Rosalind E, Park B Kevin, Polański Radoslaw, Vlatković Nikolina, Boyd Mark T (May. 2008). "MDM2 regulates dihydrofolate reductase activity through monoubiquitination". Cancer Res. (United States) 68 (9): 3232–42. doi:10.1158/0008-5472.CAN-07-5271. PMID 18451149.
- ^ The interactive pathway map can be edited at WikiPathways: "FluoropyrimidineActivity_WP1601". http://www.wikipathways.org/index.php/Pathway:WP1601.
Further reading
- Joska TM, Anderson AC (October 2006). "Structure-activity relationships of Bacillus cereus and Bacillus anthracis dihydrofolate reductase: toward the identification of new potent drug leads". Antimicrob. Agents Chemother. 50 (10): 3435–43. doi:10.1128/AAC.00386-06. PMC 1610094. PMID 17005826. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1610094.
- Chan DC, Fu H, Forsch RA, Queener SF, Rosowsky A (June 2005). "Design, synthesis, and antifolate activity of new analogues of piritrexim and other diaminopyrimidine dihydrofolate reductase inhibitors with omega-carboxyalkoxy or omega-carboxy-1-alkynyl substitution in the side chain". J. Med. Chem. 48 (13): 4420–31. doi:10.1021/jm0581718. PMID 15974594.
- Banerjee D, Mayer-Kuckuk P, Capiaux G, et al. (2002). "Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase.". Biochim. Biophys. Acta 1587 (2-3): 164–73. doi:10.1016/S0925-4439(02)00079-0. PMID 12084458.
- Stockman BJ, Nirmala NR, Wagner G, et al. (1992). "Sequence-specific 1H and 15N resonance assignments for human dihydrofolate reductase in solution.". Biochemistry 31 (1): 218–29. doi:10.1021/bi00116a031. PMID 1731871.
- Beltzer JP, Spiess M (1991). "In vitro binding of the asialoglycoprotein receptor to the beta adaptin of plasma membrane coated vesicles.". EMBO J. 10 (12): 3735–42. PMC 453108. PMID 1935897. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=453108.
- Davies JF, Delcamp TJ, Prendergast NJ, et al. (1991). "Crystal structures of recombinant human dihydrofolate reductase complexed with folate and 5-deazafolate.". Biochemistry 29 (40): 9467–79. doi:10.1021/bi00492a021. PMID 2248959.
- Will CL, Dolnick BJ (1990). "5-Fluorouracil inhibits dihydrofolate reductase precursor mRNA processing and/or nuclear mRNA stability in methotrexate-resistant KB cells.". J. Biol. Chem. 264 (35): 21413–21. PMID 2592384.
- Masters JN, Attardi G (1985). "Discrete human dihydrofolate reductase gene transcripts present in polysomal RNA map with their 5' ends several hundred nucleotides upstream of the main mRNA start site.". Mol. Cell. Biol. 5 (3): 493–500. PMC 366741. PMID 2859520. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=366741.
- Miszta H, Dabrowski Z, Lanotte M (1988). "In vitro patterns of enzymic tetrahydrofolate dehydrogenase (EC 1.5.1.3) expression in bone marrow stromal cells.". Leukemia 2 (11): 754–9. PMID 3185016.
- Oefner C, D'Arcy A, Winkler FK (1988). "Crystal structure of human dihydrofolate reductase complexed with folate.". Eur. J. Biochem. 174 (2): 377–85. doi:10.1111/j.1432-1033.1988.tb14108.x. PMID 3383852.
- Yang JK, Masters JN, Attardi G (1984). "Human dihydrofolate reductase gene organization. Extensive conservation of the G + C-rich 5' non-coding sequence and strong intron size divergence from homologous mammalian genes.". J. Mol. Biol. 176 (2): 169–87. doi:10.1016/0022-2836(84)90419-4. PMID 6235374.
- Masters JN, Yang JK, Cellini A, Attardi G (1983). "A human dihydrofolate reductase pseudogene and its relationship to the multiple forms of specific messenger RNA.". J. Mol. Biol. 167 (1): 23–36. doi:10.1016/S0022-2836(83)80032-1. PMID 6306253.
- Chen MJ, Shimada T, Moulton AD, et al. (1984). "The functional human dihydrofolate reductase gene.". J. Biol. Chem. 259 (6): 3933–43. PMID 6323448.
- Funanage VL, Myoda TT, Moses PA, Cowell HR (1985). "Assignment of the human dihydrofolate reductase gene to the q11----q22 region of chromosome 5.". Mol. Cell. Biol. 4 (10): 2010–6. PMC 369017. PMID 6504041. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=369017.
- Masters JN, Attardi G (1983). "The nucleotide sequence of the cDNA coding for the human dihydrofolic acid reductase.". Gene 21 (1-2): 59–63. doi:10.1016/0378-1119(83)90147-6. PMID 6687716.
- Morandi C, Masters JN, Mottes M, Attardi G (1982). "Multiple forms of human dihydrofolate reductase messenger RNA. Cloning and expression in Escherichia coli of their DNA coding sequence.". J. Mol. Biol. 156 (3): 583–607. doi:10.1016/0022-2836(82)90268-6. PMID 6750132.
- Bonifaci N, Sitia R, Rubartelli A (1996). "Nuclear translocation of an exogenous fusion protein containing HIV Tat requires unfolding.". AIDS 9 (9): 995–1000. doi:10.1097/00002030-199509000-00003. PMID 8527095.
- Mayhew M, da Silva AC, Martin J, et al. (1996). "Protein folding in the central cavity of the GroEL-GroES chaperonin complex.". Nature 379 (6564): 420–6. doi:10.1038/379420a0. PMID 8559246.
- Gross M, Robinson CV, Mayhew M, et al. (1997). "Significant hydrogen exchange protection in GroEL-bound DHFR is maintained during iterative rounds of substrate cycling.". Protein Sci. 5 (12): 2506–13. doi:10.1002/pro.5560051213. PMC 2143321. PMID 8976559. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2143321.
- Schleiff E, Shore GC, Goping IS (1997). "Human mitochondrial import receptor, Tom20p. Use of glutathione to reveal specific interactions between Tom20-glutathione S-transferase and mitochondrial precursor proteins.". FEBS Lett. 404 (2-3): 314–8. doi:10.1016/S0014-5793(97)00145-2. PMID 9119086.
- Cody V, Galitsky N, Luft JR, et al. (1997). "Comparison of two independent crystal structures of human dihydrofolate reductase ternary complexes reduced with nicotinamide adenine dinucleotide phosphate and the very tight-binding inhibitor PT523.". Biochemistry 36 (45): 13897–903. doi:10.1021/bi971711l. PMID 9374868.
- Vanguri VK, Wang S, Godyna S, et al. (2001). "Thrombospondin-1 binds to polyhistidine with high affinity and specificity.". Biochem. J. 347 (Pt 2): 469–73. doi:10.1042/0264-6021:3470469. PMC 1220979. PMID 10749676. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1220979.
PDB gallery 1boz: STRUCTURE-BASED DESIGN AND SYNTHESIS OF LIPOPHILIC 2,4-DIAMINO-6-SUBSTITUTED QUINAZOLINES AND THEIR EVALUATION AS INHIBITORS OF DIHYDROFOLATE REDUCTASE AND POTENTIAL ANTITUMOR AGENTS1dhf: CRYSTAL STRUCTURES OF RECOMBINANT HUMAN DIHYDROFOLATE REDUCTASE COMPLEXED WITH FOLATE AND 5-DEAZOFOLATE1dlr: METHOTREXATE-RESISTANT VARIANTS OF HUMAN DIHYDROFOLATE REDUCTASE WITH SUBSTITUTION OF LEUCINE 22: KINETICS, CRYSTALLOGRAPHY AND POTENTIAL AS SELECTABLE MARKERS1dls: METHOTREXATE-RESISTANT VARIANTS OF HUMAN DIHYDROFOLATE REDUCTASE WITH SUBSTITUTION OF LEUCINE 22: KINETICS, CRYSTALLOGRAPHY AND POTENTIAL AS SELECTABLE MARKERS1drf: CRYSTAL STRUCTURE OF HUMAN DIHYDROFOLATE REDUCTASE COMPLEXED WITH FOLATE1hfp: COMPARISON OF TERNARY CRYSTAL COMPLEXES OF HUMAN DIHYDROFOLATE REDUCTASE WITH NADPH AND A CLASSICAL ANTITUMOR FUROPYRIMDINE1hfq: COMPARISON OF TERNARY CRYSTAL COMPLEXES OF HUMAN DIHYDROFOLATE REDUCTASE WITH NADPH AND A CLASSICAL ANTITUMOR FUROPYRIMDINE1hfr: COMPARISON OF TERNARY CRYSTAL COMPLEXES OF HUMAN DIHYDROFOLATE REDUCTASE WITH NADPH AND A CLASSICAL ANTITUMOR FUROPYRIMDINE1kms: HUMAN DIHYDROFOLATE REDUCTASE COMPLEXED WITH NADPH AND 6-([5-QUINOLYLAMINO]METHYL)-2,4-DIAMINO-5-METHYLPYRIDO[2,3-D]PYRIMIDINE (SRI-9439), A LIPOPHILIC ANTIFOLATE1kmv: HUMAN DIHYDROFOLATE REDUCTASE COMPLEXED WITH NADPH AND (Z)-6-(2-[2,5-DIMETHOXYPHENYL]ETHEN-1-YL)-2,4-DIAMINO-5-METHYLPYRIDO[2,3-D]PYRIMIDINE (SRI-9662), A LIPOPHILIC ANTIFOLATE1mvs: Analysis of Two Polymorphic Forms of a Pyrido[2,3-d]pyrimidine N9-C10 Reverse-Bridge Antifolate Binary Complex with Human Dihydrofolate Reductase1mvt: Analysis of Two Polymorphic Forms of a Pyrido[2,3-d]pyrimidine N9-C10 Reverse-Bridge Antifolate Binary Complex with Human Dihydrofolate Reductase1ohj: HUMAN DIHYDROFOLATE REDUCTASE, MONOCLINIC (P21) CRYSTAL FORM1ohk: HUMAN DIHYDROFOLATE REDUCTASE, ORTHORHOMBIC (P21 21 21) CRYSTAL FORM1pd8: Analysis of Three Crystal Structure Determinations of a 5-Methyl-6-N-Methylanilino Pyridopyrimidine Antifolate Complex with Human Dihydrofolate Reductase1pd9: Analysis of Three Crystal Structure Determinations of a 5-Methyl-6-N-Methylanilino Pyridopyrimidine antifolate Complex with Human Dihydrofolate Reductase1pdb: Analysis of Three Crystal Structure Determinations of a 5-Methyl-6-N-Methylanilino Pyridopyrimidine Antifolate Complex with Human Dihydrofolate Reductase1s3u: Structure Determination of Tetrahydroquinazoline Antifolates in Complex with Human and Pneumocystis carinii Dihydrofolate Reductase: Correlations of Enzyme Selectivity and Stereochemistry1s3v: Structure Determination of Tetrahydroquinazoline Antifolates in Complex with Human and Pneumocystis carinii Dihydrofolate Reductase: Correlations of Enzyme Selectivity and Stereochemistry1s3w: Structure Determination of Tetrahydroquinazoline Antifoaltes in Complex with Human and Pneumocystis carinii Dihydrofolate Reductase: Correlations of Enzyme Selectivity and Stereochemistry1u71: Understanding the Role of Leu22 Variants in Methotrexate Resistance: Comparison of Wild-type and Leu22Arg Variant Mouse and Human Dihydrofolate Reductase Ternary Crystal Complexes with Methotrexate and NADPH1u72: Understanding the Role of Leu22 Variants in Methotrexate Resistance: Comparison of Wild-type and Leu22Arg Variant Mouse and Human Dihydrfolate Reductase Ternary Crystal Complexes with Methotrexate and NADPH1yho: Solution structure of human dihydrofolate reductase complexed with trimethoprim and nadph, 25 structures2c2s: HUMAN DIHYDROFOLATE REDUCTASE COMPLEXED WITH NADPH AND 2,4-DIAMINO-5-(1-O-CARBORANYLMETHYL)-6-METHYLPYRIMIDINE, A NOVEL BORON CONTAINING, NONCLASSICAL ANTIFOLATE2c2t: HUMAN DIHYDROFOLATE REDUCTASE COMPLEXED WITH NADPH AND 2,4-DIAMINO-5-((7,8-DICARBAUNDECABORAN-7-YL)METHYL)-6-METHYLPYRIMIDINE, A NOVEL BORON CONTAINING, NONCLASSICAL ANTIFOLATE2dhf: CRYSTAL STRUCTURES OF RECOMBINANT HUMAN DIHYDROFOLATE REDUCTASE COMPLEXED WITH FOLATE AND 5-DEAZOFOLATEExternal links
Oxidoreductases: CH-NH (EC 1.5) 1.5.1: NAD or NADP acceptor 1.5.3: oxygen acceptor 1.5.5: quinone acceptor 1.5.99 B enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 Metabolism of vitamins, coenzymes, and cofactors Fat soluble vitamins Alpha-tocopherol transfer proteinliver (Sterol 27-hydroxylase or CYP27A1) · renal (25-Hydroxyvitamin D3 1-alpha-hydroxylase or CYP27B1) · degradation (1,25-Dihydroxyvitamin D3 24-hydroxylase or CYP24A1)Water soluble vitamins Dihydropteroate synthase · Dihydrofolate reductase · Serine hydroxymethyltransferase
Methylenetetrahydrofolate reductaseNonvitamin cofactors M: NUT
cof, enz, met
noco, nuvi, sysi/epon, met
drug(A8/11/12)
Purine metabolism AnabolismR5P->IMP: Ribose-phosphate diphosphokinase · Amidophosphoribosyltransferase · Phosphoribosylglycinamide formyltransferase · AIR synthetase (FGAM cyclase) · Phosphoribosylaminoimidazole carboxylase · Phosphoribosylaminoimidazolesuccinocarboxamide synthase · IMP synthase
IMP->AMP: Adenylosuccinate synthase · Adenylosuccinate lyase · reverse (AMP deaminase)
IMP->GMP: IMP dehydrogenase · GMP synthase · reverse (GMP reductase)CatabolismPyrimidine metabolism AnabolismCatabolismDeoxyribonucleotides Ribonucleotide reductase · Nucleoside-diphosphate kinase · DCMP deaminase · Thymidylate synthase · Dihydrofolate reductaseThis article includes text from the public domain Pfam and InterPro IPR001796
This article includes text from the public domain Pfam and InterPro IPR009159
Categories:- Human proteins
- Protein domains
- EC 1.5.1
- Enzymes
Wikimedia Foundation. 2010.