- Molybdenum cofactor
-
Molybdenum cofactor 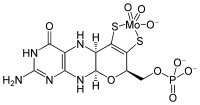 Other namesMoco
Other namesMocoIdentifiers CAS number 872689-63-9 
PubChem 25202532 DrugBank DB02137 Jmol-3D images Image 1 - [S-]C1=C([S-])C(N2)C(OC1COP(O)(O)=O)NC3=C2C(N=C(N)N3)=O.O=[Mo+2]=O
Properties Molecular formula C10H12MoN5O8PS2 Molar mass 521.27 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Molybdenum cofactor is a cofactor required for the activity of enzymes such as sulfite oxidase, xanthine oxidoreductase, and aldehyde oxidase.[1][2] It is a coordination complex formed between molybdopterin (which, despite the name, does not contain molybdenum) and an oxide of molybdenum.
Molybdopterins, in turn, are synthesized from guanosine triphosphate (see synthetic route at right).
Molybdenum cofactor functions directly in ethylbenzene dehydrogenase, glyceraldehyde-3-phosphate ferredoxin oxidoreductase, and respiratory arsenate reductase
In animals and plants these enzymes use molybdenum bound at the active site in a tricyclic molybdenum cofactor. All molybdenum-using enzymes so far identified in nature use this cofactor, save for the phylogenetically ancient molybdenum nitrogenases, which fix nitrogen in some bacteria and cyanobacteria.[3] Molybdenum enzymes in plants and animals catalyze the oxidation and sometimes reduction of certain small molecules, as part of the regulation of nitrogen, sulfur and carbon cycles.[4]
See also
- Molybdenum cofactor deficiency, a genetic illness.
- MOCS1, MOCS2, MOCS3, GEPH
References
- ^ Schwarz G (December 2005). "Molybdenum cofactor biosynthesis and deficiency". Cell. Mol. Life Sci. 62 (23): 2792–810. doi:10.1007/s00018-005-5269-y. PMID 16261263.
- ^ Smolinsky B, Eichler SA, Buchmeier S, Meier JC, Schwarz G (June 2008). "Splice-specific functions of gephyrin in molybdenum cofactor biosynthesis". J. Biol. Chem. 283 (25): 17370–9. doi:10.1074/jbc.M800985200. PMID 18411266.
- ^ [1] Structure, synthesis, empirical formula for the di-sulfhydryl. Accessed Nov. 16, 2009.
- ^ Kisker, C.; Schindelin, H.; Baas, D.; Rétey, J.; Meckenstock, R.U; Kroneck, P.M.H (1999). "A structural comparison of molybdenum cofactor-containing enzymes". FEMS Microbiol. Rev. 22 (5): 503–521. doi:10.1111/j.1574-6976.1998.tb00384.x. PMID 9990727.
Enzyme cofactors Active forms TPP / ThDP (B1) · FMN, FAD (B2) · NAD+, NADH, NADP+, NADPH (B3) · Coenzyme A (B5) · PLP / P5P (B6) · Biotin (B7) · THFA / H4FA, DHFA / H2FA, MTHF (B9) · AdoCbl, MeCbl (B12) · Ascorbic Acid (C) · Phylloquinone (K1), Menaquinone (K2) · Coenzyme F420ATP · CTP · SAMe · PAPS · GSH · Coenzyme B · Cofactor F430 · Coenzyme M · Coenzyme Q · Heme / Haem (A, B, C, O) · Lipoic Acid · Methanofuran · Molybdopterin/Molybdenum cofactor · PQQ · THB / BH4 · THMPT / H4MPTBase forms M: NUT
cof, enz, met
noco, nuvi, sysi/epon, met
drug(A8/11/12)
Categories:- Cofactors
- Molybdenum heterocycles
- Organophosphates
- Nitrogen heterocycles
- Oxygen heterocycles
- Sulfur heterocycles
- Biochemistry stubs
Wikimedia Foundation. 2010.

