- Coniferyl alcohol
-
Coniferyl alcohol 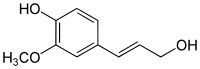 4-(3-hydroxy-1-propenyl)-
4-(3-hydroxy-1-propenyl)-
2-methoxyphenolOther names4-hydroxy-3-methoxycinnamyl alcoholIdentifiers CAS number 458-35-5 
PubChem 1549095 ChemSpider 1266063 
ChEBI CHEBI:17745 
ChEMBL CHEMBL501870 
Jmol-3D images Image 1 - Oc1ccc(cc1OC)/C=C/CO
Properties Molecular formula C10H12O3 Molar mass 180.2 g mol−1 Melting point 74 °C
Boiling point 163-165 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Coniferyl alcohol is an organic compound. This colourless crystalline solid is a phytochemical, one of the monolignols. It is synthethized via the phenylpropanoid biochemical pathway. When copolymerized with related aromatic compounds, coniferyl alcohol forms lignin or lignans.[1] Coniferin is a glucoside of coniferyl alcohol.
Coniferyl alcohol is an intermediate in biosynthesis of eugenol and of stilbenoids and coumarin. Gum benzoin contains significant amount of coniferyl alcohol and its esters.
It is found in both gymnosperm and angiosperm plants. Sinapyl alcohol and paracoumaryl alcohol, the other two lignin monomers, are found in angiosperm plants and grasses.
It is a queen retinue pheromone (QRP), a type of honey bee pheromone found in the mandibular glands.[2]
References
- ^ Kenji liyama, Thi Bach-Tuyet Lam, and Bruce A. Stone (1994). "Covalent Cross-Links in the Cell Wall". Plant Physiology 104 (2): 315–320. PMC 159201. PMID 12232082. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=159201.
- ^ Keeling, C. I., Slessor, K. N., Higo, H. A. and Winston, M. L. (2003) Isolation and identification of new components of the honey bee (Apis mellifera L.) queen retinue pheromone. PNAS, April 15, 2003 vol. 100 no. 8 4486-4491, doi:10.1073/pnas.0836984100
Aglycones Glycosides Phenylpropanoids Hydroxycinnamic acids | Chromones (Furanochromones) | Cinnamaldehydes | Monolignols | Coumarins | Flavonoids | Phenylpropenes | Stilbenoids | Lignans | Lignins | SuberinsCategories:- Phenylpropanoids
- Monolignols
Wikimedia Foundation. 2010.
