- Hepatitis C
-
Hepatitis C Classification and external resources 
Electron micrograph of hepatitis C virus purified from cell culture. The scale = 50 nanometersICD-10 B17.1, B18.2 ICD-9 070.70,070.4, 070.5 OMIM 609532 DiseasesDB 5783 MedlinePlus 000284 eMedicine med/993 ped/979 MeSH D006526 Hepatitis C is an infectious disease primarily affecting the liver, caused by the hepatitis C virus (HCV).[1] The infection is often asymptomatic, but chronic infection can lead to scarring of the liver and ultimately to cirrhosis, which is generally apparent after many years. In some cases, those with cirrhosis will go on to develop liver failure or other complications, including liver cancer or life-threatening esophageal varices and gastric varices.[1]
The hepatitis C virus is spread by blood-to-blood contact. Most people have few, if any, symptoms after the initial infection, yet the virus persists in the liver in about 85% of those infected. Persistent infection can be treated with medication; peginterferon and ribavirin are the current standard therapy. Overall, between 51-80% of treated patients are cured. Those who develop cirrhosis or liver cancer may require a liver transplant, and the virus universally recurs after transplantation.
An estimated 180 million people worldwide are infected with hepatitis C. Hepatitis C is not known to cause disease in other animals. No vaccine against hepatitis C is currently available. The existence of hepatitis C (originally "non-A non-B hepatitis") was postulated in the 1970s and proven in 1989.[2]
Contents
Signs and symptoms
Acute infection
During the first 12 weeks after infection with HCV, most people suffer no symptoms. For those who do, the main manifestations of acute infection are generally mild and vague, and rarely point to a specific diagnosis of hepatitis C. Symptoms of acute hepatitis C infection include decreased appetite, fatigue, abdominal pain, jaundice, itching, and flu-like symptoms.
The hepatitis C virus is usually detectable in the blood by PCR within one to three weeks after infection, and antibodies to the virus are generally detectable within three to 15 weeks. Spontaneous viral clearance rates are highly variable; between 10 and 60%[3] of persons infected with HCV clear the virus from their bodies during the acute phase, as shown by normalization of the liver enzymes alanine transaminase (ALT) and aspartate transaminase (AST), and plasma HCV-RNA clearance (this is known as spontaneous viral clearance). However, persistent infections are common[4] and most patients develop chronic hepatitis C, i.e., infection lasting more than 6 months.[5][6][7]
Previous practice was to not treat acute infections to see if the person would spontaneously clear; recent studies have shown that treatment during the acute phase of genotype 1 infections has a greater than 90% success rate, with half the treatment time required for chronic infections.[8]
Chronic infection
Chronic hepatitis C is defined as infection with the hepatitis C virus persisting for more than six months. Clinically, it is often asymptomatic, and it is mostly discovered accidentally, following the investigation of elevated liver enzyme levels discovered during a standard checkup.
The natural course of chronic hepatitis C varies considerably from one person to another. Although almost all people infected with HCV have evidence of inflammation on liver biopsy, the rate of progression of liver scarring (fibrosis) shows significant variability among individuals. Accurate estimates of the risk over time are difficult to establish because of the limited time that tests for this virus have been available.
Recent data suggest that among untreated patients, roughly one-third progress to liver cirrhosis in less than 20 years. Another third progress to cirrhosis within 30 years. The remainder of patients appear to progress so slowly that they are unlikely to develop cirrhosis within their lifetimes. In contrast, the NIH consensus guidelines state the risk of progression to cirrhosis over a 20-year period is 3-20 percent.[9]
Factors that have been reported to influence the rate of HCV disease progression include age (increasing age associated with more rapid progression), gender (males have more rapid disease progression than females), alcohol consumption (associated with an increased rate of disease progression), HIV coinfection (associated with a markedly increased rate of disease progression), and fatty liver (the presence of fat in liver cells has been associated with an increased rate of disease progression).
Symptoms specifically suggestive of liver disease are typically absent until substantial scarring of the liver has occurred. However, hepatitis C is a systemic disease and patients may experience a wide spectrum of clinical manifestations ranging from an absence of symptoms to a more symptomatic illness prior to the development of advanced liver disease. Generalized signs and symptoms associated with chronic hepatitis C include fatigue, flu-like symptoms, joint pains, itching, sleep disturbances, appetite changes, nausea, and depression.
Once chronic hepatitis C has progressed to cirrhosis, signs and symptoms may appear that are generally caused by either decreased liver function or increased pressure in the liver circulation, a condition known as portal hypertension. Possible signs and symptoms of liver cirrhosis include ascites (accumulation of fluid in the abdomen), bruising and bleeding tendency, varices (enlarged veins, especially in the stomach and esophagus), jaundice, and a syndrome of cognitive impairment known as hepatic encephalopathy. Hepatic encephalopathy is due to the accumulation of ammonia and other substances normally cleared by a healthy liver.
Liver enzyme tests show variable elevation of ALT and AST. Periodically, they might show normal results. Usually prothrombin and albumin results are normal, but may become abnormal, once cirrhosis has developed. The levels of elevation of liver tests do not correlate well with the amount of liver injury on biopsy. Viral genotype and viral load also do not correlate with the amount of liver injury. Liver biopsy is the best test to determine the amount of scarring and inflammation. Radiographic studies, such as ultrasound or CT scan, do not always show liver injury until it is fairly advanced. However, noninvasive tests are coming, with FibroTest[10] and ActiTest, respectively estimating liver fibrosis and necrotic inflammation. These tests are validated[11] and recommended in Europe (FDA procedures initiated in USA).
Extrahepatic manifestations
Chronic hepatitis C can be associated with signs and symptoms in organs besides the liver, such as porphyria cutanea tarda, cryoglobulinemia (a form of small-vessel vasculitis)[12] and glomerulonephritis (inflammation of the kidney), specifically membranoproliferative glomerulonephritis (MPGN).[13] Hepatitis C is also rarely associated with sicca syndrome (an autoimmune disorder), thrombocytopenia, lichen planus, diabetes mellitus and with B-cell lymphoproliferative disorders.[14]
Virology
The hepatitis C virus is a small (50 nm in size), enveloped, single-stranded, positive sense RNA virus. It is the only known member of the hepacivirus genus in the family Flaviviridae. There are six major genotypes of the hepatitis C virus, which are indicated numerically (e.g., genotype 1, genotype 2, etc.). Based on the NS5 gene there are three major and eleven minor genotypes. The major genotypes diverged about 300–400 years ago from the ancestor virus. The minor genotypes diverged about 200 years ago from their major genotypes. All of the extant genotypes appear to have evolved from genotype 1 subtype 1b.[15]
The hepatitis C virus is transmitted by blood-to-blood contact. In developed countries, it is estimated that 90% of persons with chronic HCV infection were infected through transfusion of unscreened blood or blood products or via injecting drug use or sexual exposure. In developing countries, the primary sources of HCV infection are unsterilized injection equipment and infusion of inadequately screened blood and blood products. There has not been a documented transfusion-related case of hepatitis C in the United States for over a decade, as the blood supply is vigorously screened with both EIA and PCR technologies.[citation needed]
Although injection drug use is the most common route of HCV infection, any practice, activity, or situation that involves blood-to-blood exposure can potentially be a source of HCV infection. The virus may be sexually transmitted, although this is rare, and usually only occurs when an STD that causes open sores and bleeding is also present and makes blood contact more likely.[16]
Transmission
Sexual activities and practices were initially identified as potential sources of exposure to the hepatitis C virus. More recent studies question this route of transmission.[17] Currently, heterosexual vaginal intercourse is thought to be a rare means of transmission of hepatitis C infection. The following are the currently known modes of transmission. There may be other, as yet unknown, means of transmission.
Injection drug use
Those who currently use or have used drug injection as their delivery route for drugs are at increased risk for getting hepatitis C because they may be sharing needles or other drug paraphernalia (includes cookers, cotton, spoons, water, etc.), which may be contaminated with HCV-infected blood. An estimated 60% to 80% of intravenous recreational drug users in the United States have been infected with HCV.[18] Harm reduction strategies are encouraged in many countries to reduce the spread of hepatitis C, through education, provision of clean needles and syringes, and safer injecting techniques. For reasons that are not clear, transmission by this route currently appears to be declining in the USA.
The main risk factor for HCV infection is intravenous drug using. [19] Many studies showed that imprisonment is an important predictor of HBV, HCV and HIV infection. Prison conditions increase the risk of transmission of infections, including blood-borne viral infections; the risk is further increased by the use of unsterile equipment used for injection.Intravenous drug users (IDUs) are at a potential risk for acquiring blood-borne infections by parenteral and sexual routes.[20]
During the VA Testimony before the Subcommittee on Benefits Committee on Veterans’ Affairs, U.S. House of Representatives on April 13 2000, Gary A. Roselle, M. D., Program Director for Infectious Diseases, Veterans Health Administration, Department of Veterans Affairs, stated "One in 10 US Veterans are infected with HCV, a rate five times greater than the 1.8% infection rate of the general population." A study conducted in 1999 by the Veterans Health Administration (VHA) involving 26,000 veterans showed that up to 10% of all veterans in the VHA system tested positive for hepatitis C.Of the total number of persons who were hepatitis C antibody positive, and reported an era of service, 62.7% were noted to be from the Vietnam War. The second most frequent group is listed as post-Vietnam War at 18.2%, followed by 4.8% Korean War, 4.3% post-Korean War, 4.2% from WWII, and 2.7% Persian Gulf era veterans.
Blood products
Blood transfusion, blood products, or organ transplantation prior to implementation of HCV screening (in the U.S., this would refer to procedures prior to 1992) are all risk factors for hepatitis C.
A cDNA clone from the hepatitis C virus genome was first isolated in 1989[21] and reliable tests to screen for the virus were not available until 1992. Therefore, those who received blood or blood products prior to the implementation of screening the blood supply for HCV may have been exposed to the virus. Blood products include clotting factors (taken by hemophiliacs), immunoglobulin, Rhogam, platelets, and plasma. In 2001, the Centers for Disease Control and Prevention reported the risk of HCV infection from a unit of transfused blood in the United States is less than one per million transfused units.
Iatrogenic medical or dental exposure
People can be exposed to HCV via inadequately or improperly sterilized medical or dental equipment. Equipment that may harbor contaminated blood if improperly sterilized includes needles or syringes, hemodialysis equipment, oral hygiene instruments, jet air guns, etc. Scrupulous use of appropriate sterilization techniques and proper disposal of used equipment can reduce the risk of iatrogenic exposure to HCV to virtually zero. Limitations in the implementation and enforcement of stringent standard precautions in public and private medical and dental facilities is known to be the primary cause of the spread of HCV in Egypt, the country with highest rate of infection in the world.[22]
Blood exposure
Occupation
People can be exposed to HCV through accidental exposure to blood through needle sticks or blood spatter to the eyes or open wounds at work. Universal precautions to protect against such accidental exposures significantly reduce the risk of exposure to HCV.
Recreation
Contact sports and other activities, such as "slam dancing" that may result in accidental blood-to-blood exposure are potential sources of exposure to HCV.[23]
Sexual exposure
Sexual transmission of HCV is considered to be rare. Studies show the risk of sexual transmission in heterosexual, monogamous relationships is extremely rare or even nil.[24][25] The government does not recommend condom use to prevent hepatitis C transmission in long-term mutually monogamous relationships. [26] However, for not well understood reasons, the risk of transmission is higher if one has multiple sex partners and condom use is recommended. [27] Vaginal penetrative sex is believed to have a lower risk of transmission than sexual practices that involve higher levels of trauma to anogenital mucosa (anal penetrative sex, fisting, or use of sex toys).[28] For these reasons, condom use is highly recommended for those who engage in anal sex play or other sex acts likely to cause bleeding or damage mucosal linings.[29]
Body piercings and tattoos
Tattooing dyes, ink pots, stylets, and piercing implements can transmit HCV-infected blood from one person to another if proper sterilization techniques are not followed. Tattoos or piercings performed either before the mid 1980s, "underground," or nonprofessionally are of particular concern, since sterile techniques in such settings may have been insufficient to prevent disease; sharing unsterilized tattooing equipment (for example, in the prison system)[30] has an obvious increased risk of acquiring HCV. The U.S. Centers for Disease Control and Prevention's position on this subject states that, "Whenever tattoos or body piercings are performed in informal settings or with nonsterile instruments, transmission of hepatitis C and other infectious diseases is possible." Despite these risks, it is rare for tattoos in an approved facility to be directly associated with HCV infection [31]
Personal care items such as razors, toothbrushes, cuticle scissors, and other manicuring or pedicuring equipment can easily be contaminated with blood. Sharing such items can potentially lead to exposure to HCV.[32] Appropriate caution should be taken regarding any medical condition which results in bleeding, such as canker sores, cold sores, and immediately after flossing.
HCV is not spread through casual contact, such as hugging, kissing, or sharing eating or cooking utensils.[33]
Vertical
Vertical transmission refers to the transmission of a communicable disease from an infected mother to her child during the birth process. Mother-to-child transmission of hepatitis C has been well described, but occurs relatively infrequently. Transmission occurs only among women who are HCV RNA positive at the time of delivery; the risk of transmission in this setting is approximately 6 out of 100. Among women who are both HCV and HIV positive at the time of delivery, the risk of transmitting HCV is increased to approximately 25 out of 100.
The risk of vertical transmission of HCV does not appear to be associated with method of delivery or breastfeeding.
Diagnosis
The diagnosis of hepatitis C is rarely made during the acute phase of the disease, because the majority of people infected experience no symptoms during this phase. Those who do experience acute phase symptoms are rarely ill enough to seek medical attention. The diagnosis of chronic phase hepatitis C is also challenging due to the absence or lack of specificity of symptoms until advanced liver disease develops, which may not occur until decades into the disease.
Chronic hepatitis C may be suspected on the basis of the medical history (particularly if there is any history of IV drug abuse or inhaled substance usage such as cocaine), a history of piercings or tattoos, unexplained symptoms, or abnormal liver enzymes or liver function tests found during routine blood testing. Occasionally, hepatitis C is diagnosed as a result of targeted screening, such as blood donation (blood donors are screened for numerous blood-borne diseases including hepatitis C) or contact tracing.
Hepatitis C testing begins with serological blood tests used to detect antibodies to HCV. Anti-HCV antibodies can be detected in 80% of patients within 15 weeks after exposure, in >90% within 5 months after exposure, and in >97% by 6 months after exposure. Overall, HCV antibody tests have a strong positive predictive value for exposure to the hepatitis C virus, but may miss patients who have not yet developed antibodies (seroconversion), or have an insufficient level of antibodies to detect. Immunocompromised individuals infected with HCV may never develop antibodies to the virus and therefore, never test positive using HCV antibody screening. Because of this possibility, RNA testing (see nucleic acid testing methods below) should be considered when antibody testing is negative but suspicion of hepatitis C is high (e.g. because of elevated transaminases in someone with risk factors for hepatitis C). However, liver function tests alone are not useful in predicting the severity of infection and normal results do not exclude the possibility of liver disease.[34]
Anti-HCV antibodies indicate exposure to the virus, but cannot determine if ongoing infection is present. All persons with positive anti-HCV antibody tests must undergo additional testing for the presence of the hepatitis C virus itself to determine whether current infection is present. The presence of the virus is tested for using molecular nucleic acid testing methods, such as polymerase chain reaction (PCR), transcription mediated amplification (TMA), or branched DNA (b-DNA). All HCV nucleic acid molecular tests have the capacity to detect not only whether the virus is present, but also to measure the amount of virus present in the blood (the HCV viral load). The HCV viral load is an important factor in determining the probability of response to interferon-based therapy, but does not indicate disease severity nor the likelihood of disease progression.
In people with confirmed HCV infection, genotype testing is generally recommended. HCV genotype testing is used to determine the required length and potential response to interferon-based therapy.
Prevention
According to Centers for Disease Control, hepatitis C virus is spread by exposure to large quantities of blood, either through the skin or by injection:[35]
- Injection drug use (currently the most common means of HCV transmission in the United States)
- Receipt of donated blood, blood products, and organs (once a common means of transmission, but now rare in the United States since blood screening became available in 1992)
- Needle stick injuries in healthcare settings
- Birth to an HCV-infected mother
HCV can also be spread infrequently through
- Sex with an HCV-infected person (an inefficient means of transmission)
- Sharing personal items contaminated with infectious blood, such as razors or toothbrushes (also inefficient vectors of transmission)
- Other healthcare procedures that involve invasive procedures, such as injections (usually recognized in the context of outbreaks)
- Sharing drug products via insufflation
Strategies such as the provision of new needles and syringes, and education about safer drug injection procedures, greatly decrease the risk of hepatitis C spreading between injecting drug users.
No vaccine protects against contracting hepatitis C, or helps to treat it. Vaccines are under development and some have shown encouraging results.[36]
Treatment
The hepatitis C virus induces chronic infection in 50%-80% of infected persons. Approximately 20-50% of these do not respond to therapy, depending on the genotype they are infected with. There is a very small chance of clearing the virus spontaneously in chronic HCV carriers (0.5% to 0.74% per year).[37][38] However, the majority of patients with chronic hepatitis C will not clear it without treatment.
In May 2011, the Food and Drug Administration approved 2 drugs for hepatitis C. The first one is boceprevir and the other is telaprevir (Incivek). Both drugs block an enzyme that helps the virus reproduce. The drugs are intended to improve on standard treatments using the injected drug pegylated interferon alpha and the pill ribavirin.[39]
Medications
Treatment is generally recommended for patients with proven hepatitis C virus infection and persistently abnormal liver function tests. Current treatment is a combination of pegylated interferon-alpha-2a or pegylated interferon-alpha-2b (brand names Pegasys or PEG-Intron) and the antiviral drug ribavirin for a period of 24 or 48 weeks, depending on hepatitis C virus genotype. In a large multicenter randomized control study among genotype 2 or 3 infected patients (NORDymanIC),[40] patients achieving HCV RNA below 1000 IU/mL by day 7 who were treated for 12 weeks demonstrated similar cure rates as those treated for 24 weeks.[41][42]
Pegylated interferon-alpha-2a plus ribavirin may increase sustained virological response among patients with chronic hepatitis C as compared to pegylated interferon-alpha-2b plus ribavirin according to a systematic review of randomized controlled trials .[43] The relative benefit increase was 14.6%. For patients at similar risk to those in this study (41.0% had sustained virological response when not treated with pegylated interferon alpha 2a plus ribavirin), this leads to an absolute benefit increase of 6%. About 16.7 people need to be treated for one to benefit.
Treatment during the acute infection phase has much higher success rates (greater than 90%) with a shorter duration of treatment; however, this must be balanced against the 15-40% chance of spontaneous clearance without treatment (see Acute Hepatitis C section above). Those with low initial viral loads respond much better to treatment than those with higher viral loads (greater than 400,000 IU/mL). Current combination therapy is usually supervised by physicians in the fields of gastroenterology, hepatology or infectious disease.
The treatment may be physically demanding, particularly for those with a prior history of drug or alcohol abuse. It can qualify for temporary disability in some cases. A substantial proportion of people will experience a panoply of side effects ranging from a 'flu-like' syndrome (the most common, experienced for a few days after the weekly injection of interferon) to severe adverse events including anemia, cardiovascular events and psychiatric problems such as suicide or suicidal ideation. The latter are exacerbated by the general physiological stress experienced by the patient.
Boceprevir
Boceprevir is a protease inhibitor that binds to the HCV nonstructural 3 (NS3) active site on hepatitis C genotype 1. There have been several recent randomized double-blinded clinical trials studying boceprevir in conjunction with peginterferon-ribavirin as therapy for untreated chronic HCV genotype 1 infection[44] and previously treated chronic HCV genotype 1 infection.[45] These studies have shown improved sustained virologic response at 44 weeks compared to therapy with peginterferon-ribavirin therapy alone. Anemia was a common side effect in these two studies.
Boceprevir was approved by the FDA on May 13, 2011 for the treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin. The indication is for adult patients (18 years of age and older) with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy.[46]
Cure rates by genotype
Responses can vary by genotype. Approximately 80% of hepatitis C patients in the United States have genotype 1, and genotype 4 is more common in the Middle East and Africa.
Genotype Description 2 and 3 Sustained cure rates (sustained viral response) of 75% or better are seen in people with HCV genotypes 2 and 3 with 24 weeks of treatment.[47] Patients achieving HCV RNA below 1000 IU/mL by day 7 (i.e. just prior to the second dose of pegylated interferon) may be treated for as little as 12 weeks with retained sustained cure rates.[41] 1 Sustained response is about 50% in patients with HCV genotype 1 given 48 weeks of treatment. In patients with HCV genotype 1, if treatment with pegylated interferon + ribavirin does not produce a 2-log viral load reduction or complete clearance of RNA (termed "early virological response") after 12 weeks the chance of treatment success is less than 1%. 4 Sustained response is about 65% in those with genotype 4 given 48 weeks of treatment. 6 The evidence for treatment in genotype 6 disease is currently sparse, and the evidence that exists is for 48 weeks of treatment at the same doses as are used for genotype 1 disease.[48] Physicians considering shorter durations of treatment (e.g., 24 weeks) should do so within the context of a clinical trial. Early virological response is typically not tested in non-genotype 1 patients, as the chances of attaining it are greater than 90%. The mechanism of cure is not entirely clear, because some patients who have a sustained virological response still appear to have actively replicating virus in their liver and peripheral blood mononuclear cells.[49]
Host factors
For genotype 1 hepatitis C treated with pegylated interferon-alpha-2a or pegylated interferon-alpha-2b combined with ribavirin, it has been shown that genetic polymorphisms near the human IL28B gene, encoding interferon lambda 3, are associated with significant differences in response to the treatment. This finding, originally reported in Nature,[50] showed that genotype 1 hepatitis C patients carrying certain genetic variant alleles near the IL28B gene are more likely to achieve sustained virological response after the treatment than others. A later report from Nature [51] demonstrated the same genetic variants are also associated with the natural clearance of the genotype 1 hepatitis C virus. It has subsequently been reported that polymorphisms in IL28B are strongly associated with the elimination of HCV RNA during the first days of peginterferon-α/ribavirin therapy (“first phase decline”), irrespective of HCV genotype.[52]
Similarly, baseline pretreatment plasma levels of IP-10 (also known as CXCL10) are elevated in patients chronically infected with hepatitis C virus (HCV) of genotypes 1 or 4 who do not achieve a sustained viral response (SVR) after completion of antiviral therapy.[53][54] IP-10 in plasma is mirrored by intrahepatic IP-10 mRNA, and both strikingly predict the first first phase decline during interferon/ribavirin therapy for all HCV genotypes.[55] And combining both pre-treatment levels of IP-10 and IL28B polymorphism further improves prognostication of therapeutic outcome.[56]
Increased levels of ferritin pre treatment seem to be associated with a poor response to treatment.[57]
Pregnancy and breastfeeding
If a woman who is pregnant has risk factors for hepatitis C, she should be tested for antibodies against HCV. About 4% infants born to HCV-infected women become infected. While there is no preventative treatment, there is a high probability of the baby clearing the infection in the first 12 months.
In a mother who also has HIV, the rate of transmission can be as high as 19%. There are currently no data to determine whether antiviral therapy reduces perinatal transmission. Ribavirin and interferons are contraindicated during pregnancy. However, avoiding fetal scalp monitoring and prolonged labor after rupture of membranes may reduce the risk of transmission to the infant.
HCV antibodies from the mother may persist in infants until 15 months of age. If an early diagnosis is desired, testing for HCV RNA can be performed between the ages of 2 and 6 months, with a repeat test done independent of the first test result. If a later diagnosis is preferred, an anti-HCV test can be performed after 15 months of age. Most infants infected with HCV at the time of birth have no symptoms and do well during childhood. There is no evidence that breast-feeding spreads HCV. To be cautious, an infected mother should avoid breastfeeding if her nipples are cracked and bleeding.[58]
Alternative medicine
Several alternative therapies are claimed by their proponents to be helpful for hepatitis C, or are being researched to see if they can be effective treatments. Among them are milk thistle, ginseng, colloidal silver, licorice root (or its extract glycyrrhizin), lactoferrin, TJ-108 (a mixture of herbs used in Japanese Kampo medicine), schisandra, and oxymatrine (an extract from the sophora root).[59]
In March 2011, the United States National Center for Complementary and Alternative Medicine (NCCAM) wrote:
- A review of the scientific evidence on complementary and alternative medicine (CAM) and hepatitis C found the following:
- No CAM treatment has been scientifically proven to successfully treat hepatitis C.
- A 2003 analysis of results from 13 clinical trials testing the effects of various medicinal herbs on hepatitis C concluded that there is not enough evidence to support using herbs to treat the disease.
- Two other reviews that covered a variety of CAM modalities for hepatitis C concluded that conventional therapies are the only scientifically proven treatments for the disease.
- In a 2002 NIH consensus statement on the management of hepatitis C, a panel of medical and scientific experts concluded that "alternative and nontraditional medicines" should be studied. Participants in a 2001 NIH research workshop on the benefits and risks of CAM therapies for chronic liver disease recommended research support for related laboratory and clinical studies.[59]
Additional recommendations
Current guidelines strongly recommend that hepatitis C patients be vaccinated for hepatitis A and B if they have not yet been exposed to these viruses, as infection with a second virus could worsen their liver disease.
Alcoholic beverage consumption accelerates HCV associated fibrosis and cirrhosis, and makes liver cancer more likely; insulin resistance and metabolic syndrome may similarly worsen the hepatic prognosis. There is also evidence that smoking increases the fibrosis (scarring) rate.
Special groups
Hemophilia and thalassemia are special group in HCV infected patients. Using the ribavirin in thalassemia group is not approved by FDA yet and hemophilia is at higher risk of complication in therapy with standard drugs. [60][61]
Epidemiology
 Disability-adjusted life year for hepatitis C per 100,000 inhabitants.
Disability-adjusted life year for hepatitis C per 100,000 inhabitants. no data≤ 55-1010-2020-3030-4040-5050-6060-7575-100100-150150-200≥ 200
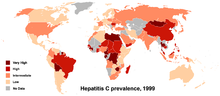
no data≤ 55-1010-2020-3030-4040-5050-6060-7575-100100-150150-200≥ 200Worldwide, it is estimated that 130-170 million people are living with chronic hepatitis C infection (~3% of the world's population), that it infects 3-4 million people per year, >10% of these people will develop liver cirrhosis or cancer over time and that more than 350,000 people die from hepatitis C related diseases each year. Countries with particularly high rates of infection include Egypt (22%), Pakistan (4.8%) and China (3.2%).[62] There are about 35,000 to 185,000 new cases a year in the United States. It is currently a leading cause of cirrhosis, a common cause of hepatocellular carcinoma, and as a result of these conditions it is the leading reason for liver transplantation in the United States. Coinfection with HIV is common, and rates among HIV positive populations are higher. Annual deaths from HCV in the United States range from 10,000 to 20,000; expectations are that this mortality rate will increase, as those who were infected by transfusion before HCV testing become apparent. A survey conducted in California showed a prevalence of up to 34% among prison inmates;[63] 82% of subjects diagnosed with hepatitis C have previously been in jail,[64] and transmission while in prison is well described.[65]
Prevalence is higher in some countries in Africa and Asia.[66] Egypt has the highest seroprevalence for HCV, up to 20% in some areas. There is a hypothesis that the high prevalence is linked to a now-discontinued mass-treatment campaign for schistosomiasis, which is endemic in that country.[67] Regardless of how the epidemic started, a high rate of HCV transmission continues in Egypt, both iatrogenically and within the community and household.
Coinfection with HIV
Approximately 350,000 people (35% of patients) in the USA infected with HIV are coinfected with the hepatitis C virus, mainly because both viruses are blood-borne and are present in similar populations. HCV is the leading cause of chronic liver disease in the USA. It has been demonstrated in clinical studies that HIV infection causes a more rapid progression of chronic hepatitis C to cirrhosis and liver failure. This is not to say treatment is not an option for those living with coinfection.
In a study involving 21 HIV coinfected patients (DICO),[68] pretreatment baseline plasma levels of IP-10 predicted the reduction of HCV RNA during the first days of interferon/ribavirin therapy (“first phase decline”) for HCV genotypes 1-3,[69] as is also the case in HCV monoinfected patients.[53][54][55] Pretreatment IP-10 levels below 150 pg/mL are predictive of a favorable response, and may thus be useful in encouraging these otherwise difficult-to-treat patients to initiate therapy.[69]
History
In the mid 1970s, Harvey J. Alter, Chief of the Infectious Disease Section in the Department of Transfusion Medicine at the National Institutes of Health, and his research team demonstrated how most post transfusion hepatitis cases were not due to hepatitis A or B viruses. Despite this discovery, international research efforts to identify the virus, initially called non-A, non-B hepatitis (NANBH), failed for the next decade. In 1987, Michael Houghton, Qui-Lim Choo, and George Kuo at Chiron Corporation, collaborating with Dr. D.W. Bradley from CDC, used a novel molecular cloning approach to identify the unknown organism and develop a diagnostic test.[70] In 1988, the virus was confirmed by Alter by verifying its presence in a panel of NANBH specimens. In April 1989, the discovery of the virus, renamed hepatitis C virus (HCV), was published in two articles in the journal Science.[71][72] The discovery led to significant improvements in diagnosis and improved antiviral treatment.[70]
Chiron filed for several patents on the virus and its diagnosis.[73] A competing patent application by the CDC was dropped in 1990 after Chiron paid $1.9 million to the CDC and $337,500 to Bradley. In 1994, Bradley sued Chiron, seeking to invalidate the patent, have himself included as a coinventor, and receive damages and royalty income. He dropped the suit in 1998 after losing before an appeals court.[74][75]
In 2000, Drs. Alter and Houghton were honored with the Lasker Award for Clinical Medical Research for "pioneering work leading to the discovery of the virus that causes hepatitis C and the development of screening methods that reduced the risk of blood transfusion-associated hepatitis in the U.S. from 30% in 1970 to virtually zero in 2000."[76]
In 2004, Chiron held 100 patents in 20 countries related to hepatitis C, and had successfully sued many companies for infringement. Scientists and competitors have complained the company hinders the fight against hepatitis C by demanding too much money for its technology.[74]
Research
The drug viramidine, which is a prodrug of ribavirin that has better targeting for the liver, and therefore may be more effective against hepatitis C for a given tolerated dose, is in phase III experimental trials against hepatitis C. It will be used in conjunction with interferons, in the same manner as ribavirin. However, this drug is not expected to be active against ribavirin-resistant strains, and the use of the drug against infections which have already failed ribavirin/interferon treatment, is unproven.
There are new drugs under development, like the protease inhibitors (including telaprevir/VX 950), entry inhibitors (such as SP 30 and ITX 5061)[77][78][79] and polymerase inhibitors (such as RG7128, PSI-7977 and NM 283), but development of some of these is still in the early phase. VX 950, also known as Telaprevir[80] is currently in Phase III trials. [81][82] One protease inhibitor, BILN 2061, had to be discontinued due to safety problems early in the clinical testing. Some more modern new drugs that provide some support in treating HCV are albuferon[83] and Zadaxin.[84] Antisense phosphorothioate oligos have been targeted to hepatitis C.[85] Antisense Morpholino oligos have shown promise in preclinical studies[86] however, they were found to cause a limited viral load reduction.[87]
Some studies have shown that HCV viral replication is dependent upon the host factor miR-122. As a result, pharmaceutical companies are developing potential HCV drugs that target miR-122. HCV therapies that target this host factor necessary for viral replication, rather than the virus itself, are promising, as they show little to no potential for viral resistance. [88] One such drug is miravirsen, developed by Santaris Pharma a/s, a locked nucleic acid based miR-122 antagonist in Phase II clinical trials as of late 2010. [89]
Immunoglobulins against the hepatitis C virus exist, and newer types are under development. Thus far, their roles have been unclear, as they have not been shown to help in clearing chronic infection or in the prevention of infection with acute exposures (e.g. needle sticks). They do have a limited role in transplant patients.
In addition to the standard treatment with interferon and ribavirin, some studies have shown higher success rates when the antiviral drug amantadine (Symmetrel) is added to the regimen. Sometimes called "triple therapy", it involves the addition of 100 mg of amantadine twice a day. Studies indicate this may be especially helpful for "nonresponders" — patients who have not been successful in previous treatments using interferon and ribavirin only.[90] Currently, amantadine is not approved for treatment of hepatitis C, and studies are ongoing to determine when it is most likely to benefit the patient and when it is a risk due to their liver deterioration.
Bristol-Myers Squibb has obtained promising Phase II results for its experimental drug, BMS-790052, an NS5A replication complex inhibitor,[91] in combination with peginterferon alfa and ribavirin.[92]
See also
- HCV-HIV coinfection
- List of people with hepatitis C
- PSI-6130 (experimental treatment)
- World Hepatitis Day
References
- ^ a b Ryan KJ, Ray CG (editors), ed (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 551–2. ISBN 0838585299.
- ^ Houghton M (November 2009). "The long and winding road leading to the identification of the hepatitis C virus". Journal of Hepatology 51 (5): 939–48. doi:10.1016/j.jhep.2009.08.004. PMID 19781804.
- ^ Caruntu FA, Benea L (September 2006). "Acute hepatitis C virus infection: Diagnosis, pathogenesis, treatment". Journal of Gastrointestinal and Liver Diseases 15 (3): 249–56. PMID 17013450. http://www.jgld.ro/32006/32006_7.html.
- ^ Kamal SM (May 2008). "Acute hepatitis C: a systematic review". The American Journal of Gastroenterology 103 (5): 1283–97; quiz 1298. doi:10.1111/j.1572-0241.2008.01825.x. PMID 18477352.
- ^ Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL (1999). "Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection". Hepatology 29 (3): 908–14. doi:10.1002/hep.510290311. PMID 10051497.
- ^ Cox AL, Netski DM, Mosbruger T, et al. (April 2005). "Prospective evaluation of community-acquired acute-phase hepatitis C virus infection". Clinical Infectious Diseases 40 (7): 951–8. doi:10.1086/428578. PMID 15824985.
- ^ "NIH Consensus Development Conference on Management of Hepatitis C: 2002". http://consensus.nih.gov/2002/2002HepatitisC2002116main.htm. Retrieved 22 February 2008.
- ^ Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, Pastore G, Dietrich M, Trautwein C, Manns MP (November 2001). "Treatment of acute hepatitis C with interferon alfa-2b". N Engl J Med 345 (20): 1452–1457. doi:10.1056/NEJMoa011232. PMID 11794193.
- ^ "NIH Consensus Statement on Management of Hepatitis C: 2002". NIH Consensus and State-of-the-science Statements 19 (3): 1–46. 2002. PMID 14768714. http://consensus.nih.gov/2002/2002HepatitisC2002116main.htm.
- ^ Ngo Y, Munteanu M, Messous D, et al. (October 2006). "A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C". Clinical Chemistry 52 (10): 1887–96. doi:10.1373/clinchem.2006.070961. PMID 16931569.
- ^ Halfon P, Munteanu M, Poynard T (September 2008). "FibroTest-ActiTest as a non-invasive marker of liver fibrosis". Gastroentérologie Clinique et Biologique 32 (6 Suppl 1): 22–39. doi:10.1016/S0399-8320(08)73991-5. PMID 18973844.
- ^ Pascual M, Perrin L, Giostra E, Schifferli JA (August 1990). "Hepatitis C virus in patients with cryoglobulinemia type II". The Journal of Infectious Diseases 162 (2): 569–70. doi:10.1093/infdis/162.2.569. PMID 2115556.
- ^ Johnson RJ, Gretch DR, Yamabe H, et al. (February 1993). "Membranoproliferative glomerulonephritis associated with hepatitis C virus infection". N Engl J Med 328 (7): 465–70. doi:10.1056/NEJM199302183280703. PMID 7678440.
- ^ Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB (January 2007). "Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach". Digestive and Liver Disease 39 (1): 2–17. doi:10.1016/j.dld.2006.06.008. PMID 16884964.
- ^ Sarwar MT, Kausar H, Ijaz B, Ahmad W, Ansar M, Sumrin A, Ashfaq UA, Asad S, Gull S, Shahid I, Hassan S (2011) NS4A protein as a marker of HCV history suggests that different HCV genotypes originally evolved from genotype 1b. Virol J 8(1):317
- ^ What is hepatitis?[dead link], Planned Parenthood, accessed May 15, 2007
- ^ Tohme RA, Holmberg SD (June 2010). "Is sexual contact a major mode of hepatitis C virus transmission?". Hepatology 52 (4): n/a. doi:10.1002/hep.23808. PMID 20635398.
- ^ "HCV Prevalence in Selected Groups of Adults by History of Injection Drug Use". http://www.cdc.gov/ncidod/diseases/hepatitis/partners/nvhpc_2005/Thursday/PL4Alter.ppt. Retrieved 3 June 2008.
- ^ Mir-Nasseri MM, Mohammadkhani A, Tavakkoli H, Ansari E, Poustchi H. Incarceration is a major risk factor for blood-borne infection among intravenous drug users. Hepat Mon. 2011;11(1):19-22.
- ^ Azarkar Z, Sharifzadeh G. Evaluation of the Prevalence of Hepatitis B, Hepatitis C, and HIV in Inmates with Drug-Related Convictions in Birjand, Iran in 2008. Hepat Mon. 2010;10(1):26-30.
- ^ Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (April 1989). "Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome". Science 244 (4902): 359–62. doi:10.1126/science.2523562. PMID 2523562.
- ^ "Highest Rates of Hepatitis C Virus Transmission Found in Egypt". Al Bawaba. 2010-08-09. http://www1.albawaba.com/en/news/highest-rates-hepatitis-c-virus-transmission-found-egypt. Retrieved 2010-08-27.
- ^ Karmochkine M, Carrat F, Dos Santos O, Cacoub P, Raguin G (November 2006). "A case-control study of risk factors for hepatitis C infection in patients with unexplained routes of infection". Journal of Viral Hepatitis 13 (11): 775–82. doi:10.1111/j.1365-2893.2006.00742.x. PMID 17052278.
- ^ Vandelli C, Renzo F, Romanò L, et al. (May 2004). "Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study". The American Journal of Gastroenterology 99 (5): 855–9. doi:10.1111/j.1572-0241.2004.04150.x. PMID 15128350.
- ^ "Hepatitis Central News". Hepatitis-central.com. 2007-04-05. http://www.hepatitis-central.com/mt/archives/2007/04/heterosexual_mo.html. Retrieved 2010-08-27.
- ^ http://www.hepatitis.va.gov/products/HCV-education-class-script.asp
- ^ http://www.hepatitis.va.gov/products/HCV-education-class-script.asp
- ^ name="Hanh2007">Hahn JA (2007). "Sex, Drugs, and Hepatitis C Virus". J Infect Dis 195 (11): 1556–9. doi:10.1086/516792. PMID 17471423.
- ^ Sex and Hepatitis C, http://sexandhepc.com/
- ^ Vescio MF, Longo B, Babudieri S, et al. (April 2008). "Correlates of hepatitis C virus seropositivity in prison inmates: a meta-analysis". Journal of Epidemiology and Community Health 62 (4): 305–13. doi:10.1136/jech.2006.051599. PMID 18339822.
- ^ http://www.cdc.gov/hepatitis/HCV/PDFs/HepCGeneralFactSheet.pdf
- ^ Lock G, Dirscherl M, Obermeier F, et al. (September 2006). "Hepatitis C — contamination of toothbrushes: myth or reality?". J. Viral Hepat. 13 (9): 571–3. doi:10.1111/j.1365-2893.2006.00735.x. PMID 16907842.
- ^ "Hepatitis C: FAQ – CDC Viral Hepatitis". http://www.cdc.gov/ncidod/diseases/hepatitis/c/faq.htm#5d. Retrieved 13 June 2008.
- ^ Shiffman ML, Diago M, Tran A, Pockros P, Reindollar R, Prati D, Rodríguez-Torres M, Lardelli P, Blotner S, Zeuzem S (May 2006). "Chronic hepatitis C in patients with persistently normal alanine transaminase levels". Clinical Gastroenterology and Hepatology 4 (5): 645–52. doi:10.1016/j.cgh.2006.02.002. PMID 16630770.
- ^ "FAQs for Health Professionals". http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section2.
- ^ Manns MP, Foster GR, Rockstroh JK, Zeuzem S, Zoulim F, Houghton M (2007). "The way forward in HCV treatment—finding the right path". Nat Rev Drug Discov 6 (12): 991–1000. doi:10.1038/nrd2411. PMID 18049473.
- ^ Watanabe H, Saito T, Shinzawa H, et al. (September 2003). "Spontaneous elimination of serum hepatitis C virus (HCV) RNA in chronic HCV carriers: a population-based cohort study". Journal of Medical Virology 71 (1): 56–61. doi:10.1002/jmv.10448. PMID 12858409.
- ^ Scott JD, McMahon BJ, Bruden D, et al. (April 2006). "High rate of spontaneous negativity for hepatitis C virus RNA after establishment of chronic infection in Alaska Natives". Clinical Infectious Diseases 42 (7): 945–52. doi:10.1086/500938. PMID 16511757.
- ^ http://yourlife.usatoday.com/health/medical/story/2011/05/FDA-clears-Vertexs-hepatitis-C-drug-Incivek/47493216/1?csp=34news&utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+usatoday-NewsTopStories+%28News+-+Top+Stories%29
- ^ Lagging M, Langeland N, Pedersen C, et al. (June 2008). "Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection". Hepatology 47 (6): 1837–45. doi:10.1002/hep.22253. PMID 18454508.
- ^ a b Lagging M, Langeland N, Pedersen C, et al. (August 2008). "Weight-adjusted dosing of ribavirin and importance of hepatitis C virus RNA below 1000 IU/mL by day 7 in short-term peginterferon therapy for chronic genotype 2/3 hepatitis C virus infection". Hepatology 48 (2): 695. doi:10.1002/hep.22450. PMID 18666232.
- ^ Lagging M, Wejstål R, Uhnoo I, et al. (2009). "Treatment of hepatitis C virus infection: updated Swedish Consensus recommendations". Scandinavian Journal of Infectious Diseases 41 (6–7): 389–402. doi:10.1080/00365540902998271. PMID 20001276.
- ^ Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C (April 2010). "Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: systematic review of randomized trials". Hepatology 51 (4): 1176–84. doi:10.1002/hep.23504. PMID 20187106.
- ^ Poordad, F, et al. (March 2011). "Boceprevir for Untreated Chronic HCV Genotype 1 Infection". N Engl J Med. 364 (13): 1195–206. doi:10.1056/NEJMoa1010494. PMID 21449783.
- ^ Bacon, B, et al. (March 2011). "Boceprevir for Previously Treated Chronic HCV Genotype 1 Infection". N Engl J Med. 364 (13): 1207–17. doi:10.1056/NEJMoa1009482. PMID 21449784.
- ^ "FDA Approves Merck's VICTRELIS™ (boceprevir), First-in-Class Oral Hepatitis C Virus (HCV) Protease Inhibitor" (Press release). Merck & Co.. http://www.merck.com/newsroom/news-release-archive/prescription-medicine-news/2011_0513.html. Retrieved 2011-05-14.
- ^ Shiffman ML, Suter F, Bacon BR, et al. (July 2007). "Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3". N Engl J Med 357 (2): 124–34. doi:10.1056/NEJMoa066403. PMID 17625124.
- ^ Fung J, Lai CL, Hung I, et al. (September 2008). "Chronic hepatitis C virus genotype 6 infection: response to pegylated interferon and ribavirin". The Journal of Infectious Diseases 198 (6): 808–12. doi:10.1086/591252. PMID 18657036.
- ^ Castillo I, Rodríguez-Iñigo E, López-Alcorocho JM, Pardo M, Bartolomé J, Carreño V (November 2006). "Hepatitis C virus replicates in the liver of patients who have a sustained response to antiviral treatment". Clinical Infectious Diseases 43 (10): 1277–83. doi:10.1086/508198. PMID 17051492.
- ^ Ge D, Fellay J, Thompson AJ, et al. (September 2009). "Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance". Nature 461 (7262): 399–401. doi:10.1038/nature08309. PMID 19684573.
- ^ Thomas DL, Thio CL, Martin MP, et al. (October 2009). "Genetic variation in IL28B and spontaneous clearance of hepatitis C virus". Nature 461 (7265): 798–801. doi:10.1038/nature08463. PMID 19759533.
- ^ Bochud PY, Bibert S, Negro F, et al. (2011). "IL28B polymorphisms predict reduction of HCV RNA from the first day of therapy in chronic hepatitis C". Journal of Hepatology. doi:10.1016/j.jhep.2011.01.050. PMID 21354446.
- ^ a b Romero AI, Lagging M, Westin J, et al. (October 2006). "Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection". The Journal of Infectious Diseases 194 (7): 895–903. doi:10.1086/507307. PMID 16960776.
- ^ a b Lagging M, Romero AI, Westin J, et al. (December 2006). "IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection". Hepatology 44 (6): 1617–25. doi:10.1002/hep.21407. PMID 17133471.
- ^ a b Askarieh G, Alsiö A, Pugnale P, et al. (May 2010). "Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C". Hepatology 51 (5): 1523–30. doi:10.1002/hep.23509. PMID 20186843.
- ^ Lagging M, Askarieh G, Negro F, et al. (Feb 24 2011). Tavis, John. ed. "Response prediction in chronic hepatitis C by assessment of baseline levels of IP-10 and Il28B-related single nucleotide polymorphisms". PLoS One 6 (2): e17232. doi:10.1371/journal.pone.0017232. PMC 3044738. PMID 21390311. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3044738.
- ^ Lange CM, Kutalik Z, Morikawa K, Bibert S, Cerny A, Dollenmaier G, Dufour JF, Gerlach TJ, Heim MH, Malinverni R, Müllhaupt B, Negro F, Moradpour D, Bochud PY; the Swiss Hepatitis C Cohort Study Group (2011) Serum ferritin levels are associated with a distinct phenotype of chronic hepatitis C poorly responding to pegylated interferon-α and ribavirin therapy. Hepatology doi: 10.1002/hep.24787.
- ^ Mast EE (2004). "Mother-to-infant hepatitis C virus transmission and breastfeeding". Advances in Experimental Medicine and Biology 554: 211–6. PMID 15384578.
- ^ a b Hepatitis C and CAM: What the Science Says. NCCAM March 2011. (Retrieved 07 March 2011)
- ^ Alavian SM, Tabatabaei SV, Keshvari M, Behnava B, Miri SM, Elizee PK, et al. Peginterferon alpha-2a and ribavirin treatment of patients with haemophilia and hepatitis C virus infection: a single-centre study of 367 cases. Liver Int. 2010 Sep;30(8):1173-80.
- ^ Alavian SM, Tabatabaei SV. Treatment of chronic hepatitis C in polytransfused thalassaemic patients: a meta-analysis. J Viral Hepat. 2010 April;17(4):236-44.
- ^ "WHO Hepatitis C factsheet". 2011. http://www.who.int/mediacentre/factsheets/fs164/en/index.html. Retrieved 2011-07-13.
- ^ Ruiz JD, Molitor F, Plagenhoef JA (November 2002). "Trends in hepatitis C and HIV infection among inmates entering prisons in California, 1994 versus 1999". AIDS 16 (16): 2236–8. doi:10.1097/00002030-200211080-00023. PMID 12409752.
- ^ Campbell JV, Hagan H, Latka MH, et al. (February 2006). "High prevalence of alcohol use among hepatitis C virus antibody positive injection drug users in three US cities". Drug and Alcohol Dependence 81 (3): 259–65. doi:10.1016/j.drugalcdep.2005.07.005. PMC 2196223. PMID 16129567. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2196223.
- ^ McGovern BH, Wurcel A, Kim AY, et al. (June 2006). "Acute hepatitis C virus infection in incarcerated injection drug users". Clinical Infectious Diseases 42 (12): 1663–70. doi:10.1086/504327. PMID 16705568.
- ^ Chapter 4 — Hepatitis, Viral, Type C — Yellow Book, CDC Health Information for International Travel 2008
- ^ Frank C, Mohamed MK, Strickland GT, et al. (March 2000). "The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt". Lancet 355 (9207): 887–91. doi:10.1016/S0140-6736(99)06527-7. PMID 10752705.
- ^ Falconer K, Sandberg JK, Reichard O, Alaeus A (2009). "HCV/HIV coinfection at a large HIV outpatient clinic in Sweden: feasibility and results of hepatitis C treatment". Scandinavian Journal of Infectious Diseases 41 (11–12): 881–5. doi:10.3109/00365540903214272. PMID 19922074.
- ^ a b Falconer K, Askarieh G, Weis N, Hellstrand K, Alaeus A, Lagging M (July 2010). "IP-10 predicts the first phase decline of HCV RNA and overall viral response to therapy in patients co-infected with chronic hepatitis C virus infection and HIV". Scandinavian Journal of Infectious Diseases 42 (11–12): 100707060350094. doi:10.3109/00365548.2010.498019. PMID 20608766.
- ^ a b Boyer, JL (2001). Liver cirrhosis and its development: proceedings of the Falk Symposium 115. Springer. pp. 344. ISBN 9780792387602.
- ^ Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (April 1989). "Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome". Science 244 (4902): 359–62. doi:10.1126/science.2523562. PMID 2523562.
- ^ Kuo G, Choo QL, Alter HJ, et al. (April 1989). "An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis". Science 244 (4902): 362–4. doi:10.1126/science.2496467. PMID 2496467.
- ^ Houghton, M., Q.-L. Choo, and G. Kuo. NANBV Diagnostics and Vaccines. European Patent No. EP-0-3 18-216-A1. European Patent Office (filed 18 November 1988, published 31 May 1989).
- ^ a b Paul Elias. ""Hepatitis Drug-Maker Complaints Reviewed", The Associated Press, 27 February 2004
- ^ Daniel W. Bradley v. Chiron Corporation, 136 F. 3d 1317 (U.S. Court of Appeals for the Federal Circuit, 1998)
- ^ 2000 Winners Albert Lasker Award for Clinical Medical Research, The Lasker Foundation. Retrieved 20 February 2008.
- ^ "SP-30: A Novel Treatment for Hepatitis C". http://www.samaritanpharma.com/aids_hiv_program_sp-10T1.asp. Retrieved 2 March 2010.
- ^ "ITX-5061". http://www.itherx.com/hepatitis.html. Retrieved 2 March 2010.
- ^ "iTherX Pharmaceuticals Announces Phase 2a Proof-of-concept Trial of First Hepatitis C Virus Entry Inhibitor". http://www.hivandhepatitis.com/hep_c/news/2009/021309_a.html. Retrieved 2 March 2010.
- ^ "Telaprevir". Vrtx.com. http://www.vrtx.com/current-projects/drug-candidates/telaprevir-VX-950.html. Retrieved 2010-08-27.
- ^ Hinrichsen H, Benhamou Y, Wedemeyer H, et al. (November 2004). "Short-term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients". Gastroenterology 127 (5): 1347–55. doi:10.1053/j.gastro.2004.08.002. PMID 15521004.
- ^ Lamarre D, Anderson PC, Bailey M, et al. (November 2003). "An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus". Nature 426 (6963): 186–9. doi:10.1038/nature02099. PMID 14578911.
- ^ "Human Genome Sciences Announces Completion of Enrollment in Phase 2b Monthly-Dosing Trial of Albuferon". PR Newswire. 19 June 2009. http://sev.prnewswire.com/health-care-hospitals/20090619/PH3522819062009-1.html. Retrieved 13 July 2009. "Trial conducted by Novartis evaluating safety and efficacy of Albuferon administered every four weeks in combination with ribavirin in patients with genotypes 2 and 3 hepatitis C"
- ^ Poo JL, Sánchez Avila F, Kershenobich D, et al. (2008). "Efficacy of triple therapy with thymalfasin, peginterferon α-2a, and ribavirin for the treatment of hispanic chronic HCV nonresponders" (PDF). Ann Hepatol 7 (4): 369–75. PMID 19034238. http://www.medigraphic.com/pdfs/hepato/ah-2008/ah084j.pdf. "More recently, thymalfasin (thymosin alpha 1, Tα1, ZADAXIN, SciClone Pharmaceuticals, Inc., CA, USA) has shown efficacy in the treatment of chronic HCV infection."
- ^ Zhang H, Hanecak R, Brown-Driver V, et al. (February 1999). "Antisense oligonucleotide inhibition of hepatitis C virus (HCV) gene expression in livers of mice infected with an HCV-vaccinia virus recombinant". Antimicrobial Agents and Chemotherapy 43 (2): 347–53. PMC 89075. PMID 9925530. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=9925530.
- ^ McCaffrey AP, Meuse L, Karimi M, Contag CH, Kay MA (August 2003). "A potent and specific morpholino antisense inhibitor of hepatitis C translation in mice". Hepatology 38 (2): 503–8. doi:10.1053/jhep.2003.50330. PMID 12883495.
- ^ ClinicalTrials.gov NCT00229749 Study of AVI-4065 in Healthy Volunteers and Chronic Active HCV Patients
- ^ Elmén J, Lindow M, Schütz S, et al. (April 2008). "LNA-mediated microRNA silencing in non-human primates". Nature 452 (7189): 896–9. doi:10.1038/nature06783. PMID 18368051.
- ^ Franciscus, Alan. "Hepatitis C Treatments in Current Development," HCV Advocate (2010): 1-22.
- ^ Maynard M, Pradat P, Bailly F, et al. (March 2006). "Amantadine triple therapy for non-responder hepatitis C patients. Clues for controversies (ANRS HC 03 BITRI)". Journal of Hepatology 44 (3): 484–90. doi:10.1016/j.jhep.2005.11.038. PMID 16426697.
- ^ Gao M, Nettles RE, Belema M, et al. (May 2010). "Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect". Nature 465 (7294): 96–100. doi:10.1038/nature08960. PMID 20410884.
- ^ Lopatto, Elizabeth (17 September 2011). "Bristol-Myers’s Once-Daily Hepatitis C Drug Works in Study". Bloomberg Businessweek. http://www.businessweek.com/news/2011-09-17/bristol-myers-s-once-daily-hepatitis-c-drug-works-in-study.html.
External links
Information and resources
- CDC's Hepatitis C Fact Sheet
- Hepatitis C at the Open Directory Project
- "What I need to know about Hepatitis C". National Digestive Diseases Information Clearinghouse. May 2004. http://digestive.niddk.nih.gov/ddiseases/pubs/hepc_ez/. Retrieved 2008-09-28.
- Virus Pathogen Database and Analysis Resource (ViPR): Flaviviridae
Organizations and programs
- National Hepatitis C Program U.S. Department of Veterans Affairs
- Hepatitis C American Liver Foundation
- Hepatitis Australia Hepatitis Australia
- Hepatitis C homepage of the UK National Health Service
- National CIHR Research Training Program in Hepatitis C Training program for student researchers funded by the Canadian Institutes of Health Research.
- Hepatitis C Research Sphinx: European Union Framework7 Hepatitis C research programme
Infectious diseases · Viral systemic diseases (A80–B34, 042–079) Oncovirus Immune disorders Central
nervous systemEncephalitis/
meningitisDNA virus: JCV (Progressive multifocal leukoencephalopathy)
RNA virus: MeV (Subacute sclerosing panencephalitis) · LCV (Lymphocytic choriomeningitis) · Arbovirus encephalitis · Orthomyxoviridae (probable) (Encephalitis lethargica) · RV (Rabies) · Chandipura virus · Herpesviral meningitis · Ramsay Hunt syndrome type IIEyeCardiovascular Respiratory system/
acute viral nasopharyngitis/
viral pneumoniaDigestive system Urogenital Categories:- Hepatitis
- Viral diseases
Wikimedia Foundation. 2010.