- Inositol
-
myo-Inositol[1] 
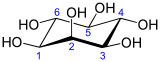 cis-1,2,3,5-trans-4,6-CyclohexanehexolOther names(1R,2R,3S,4S,5R,6S)-cyclohexane-1,2,3,4,5,6-hexol, Cyclohexanehexol,
cis-1,2,3,5-trans-4,6-CyclohexanehexolOther names(1R,2R,3S,4S,5R,6S)-cyclohexane-1,2,3,4,5,6-hexol, Cyclohexanehexol,
Mouse antialopecia factor,
Nucite, Phaseomannite,
Phaseomannitol,
Rat antispectacled eye
factor, and Scyllite
(for the structural
isomer scyllo-Inositol)Identifiers CAS number 87-89-8 
PubChem 892 ChemSpider 10239179 
UNII 4L6452S749 
KEGG D08079 
ChEBI CHEBI:17268 
ChEMBL CHEMBL1222251 
Jmol-3D images Image 1 - [C@@H]1([C@@H]([C@@H]([C@@H]([C@H]([C@@H]1O)O)O)O)O)O
Properties Molecular formula C6H12O6 Molar mass 180.16 g mol−1 Density 1.752 g/cm³ Melting point 225-227 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Inositol or cyclohexane-1,2,3,4,5,6-hexol is a chemical compound with formula C6H12O6 or (-CHOH-)6, a sixfold alcohol (polyol) of cyclohexane. It exists in nine possible stereoisomers, of which the most prominent form, widely occurring in nature, is cis-1,2,3,5-trans-4,6-cyclohexanehexol, or myo-inositol (former name meso-inositol).[2][3] Inositol is a carbohydrate, though not a classical sugar. It is almost tasteless, with a small amount of sweetness.
Myo-inositol plays an important role as the structural basis for a number of secondary messengers in eukaryotic cells, including inositol phosphates, phosphatidylinositol (PI) and phosphatidylinositol phosphate (PIP) lipids. Inositol or its phosphates and associated lipids are found in many foods, in particular fruit, especially cantaloupe and oranges.[4] In plants, the hexaphosphate of inositol, phytic acid or its salts, the phytates, are found. Phytic acid occurs also in cereals with high bran content and also nuts and beans, but inositol as phytate is not directly bioavailable to humans in the diet, since it is not digestible (some food preparation techniques partly break down phytates to change this—see phytic acid for details). Inositol as it occurs in certain plant-derived substances such as lecithins, however, is well-absorbed and relatively bioavailable.
Myo-inositol was once considered as a member of the vitamin B complex, however, because it is produced by the human body from glucose, it is not an essential nutrient.[5] Some substances such as niacin can also be synthesized in the body, but are not made in amounts considered adequate for good health, and thus are still classified as essential nutrients. However, there is no convincing evidence that this is the case for myo-inositol.
Contents
Isomers and structure
The isomer myo-inositol is a meso compound that possesses an optically inactive plane of symmetry through the molecule, and meso-inositol is an obsolete name that refers to myo-inositol. Besides myo-inositol, the other naturally occurring stereoisomers (though in minimal quantities) are scyllo-, muco-, D-chiro-, and neo-inositol. The other possible isomers are L-chiro-, allo-, epi-, and cis-inositol. As their name denotes, the two chiro inositols are the only pair of inositol enantiomers, but they are enantiomers of each other, not of myo-inositol.
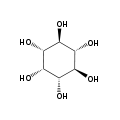
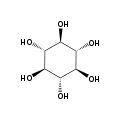
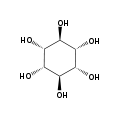
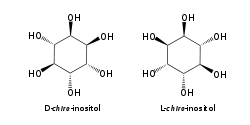
myo- scyllo- muco- chiro- 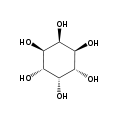
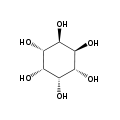
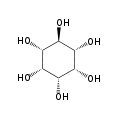
neo- allo- epi- cis- In its most stable conformational geometry, the myo-inositol isomer assumes the chair conformation, which puts the maximum number of hydroxyls to the equatorial position, where they are farthest apart from each other. In this conformation the natural myo isomer has a structure in which five of the six hydroxyls (the 1st, 3rd, 4th, 5th, and 6th are equatorial, whereas the 2nd hydroxyl group is axial.[6]
Synthesis
Myo-Inositol is synthesized from glucose-6-phosphate (G-6-P) in two steps. First, G-6-P is isomerised by an inositol-3-phosphate synthase enzyme (called ISYNA1) to myo-inositol 1-phosphate, which is then dephosphorylated by an Inositol monophosphatase enzyme (called IMPase 1) to give free myo-inositol. In humans most inositol is synthesized in the kidneys, in typical amounts of a few grams per day.[citation needed]
Function
Inositol and a number of its mono and polyphosphates function as the basis for a number of signaling and secondary messenger molecules. They are involved in a number of biological processes, including:
- Insulin signal transduction[7]
- Cytoskeleton assembly
- Nerve guidance (Epsin)
- Intracellular calcium (Ca2+) concentration control[8]
- Cell membrane potential maintenance[9]
- Serotonin activity modulation
- Breakdown of fats and reducing blood cholesterol[10]
- Gene expression[11][12]
Phytic acid in plants
Phytic acid, which is inositol hexakisphosphate (IP6), also known as phytate when in salt form, is the principal storage form of phosphorus in many plant tissues, especially bran and seeds.[13] Neither the inositol nor the phosphate in phytic acid in plants is available to humans, or to animals who are not ruminants, since it cannot be broken down, except by bacteria. Moreover, phytic acid also chelates important minerals such as calcium, magnesium, iron, and zinc, making them unabsorbable, and contributing to mineral deficiencies in people whose diets rely high bran and seed diets for their mineral intake, such as occurs in developing countries.[14][15]
Inositol penta- (IP5), tetra- (IP4), and triphosphate (IP3) are also called "phytates."
Explosive potential
At the 1936 meeting of the American Chemical Society, professor Edward Bartow of the University of Iowa presented a commercially viable means of extracting large amounts of inositol from waste corn. As a possible use for the chemical, he suggested inositol nitrate as a more stable alternative to nitroglycerin.[16] Today, inositol nitrate is used to gelatinize nitrocellulose, and thus can be found in many modern explosives and solid rocket propellants.[17]
Clinical implications
Psychiatric conditions
Some preliminary results of studies on high-dose inositol supplements show promising results for people suffering from problems such as bulimia, panic disorder, obsessive-compulsive disorder, agoraphobia, and unipolar and bipolar depression.[18][19][20][21]
In a single double-blind study on 13 patients, Myo-inositol (18 grams daily) has been found to reduce the symptoms of obsessive-compulsive disorder (OCD) significantly, with effectiveness equal to SSRIs and virtually without side-effects.[22] In a double-blind, controlled trial, myo-inositol (18 grams daily) was superior to fluvoxamine for decreasing the number of panic attacks and other side-effects.[20]
Patients suffering from clinical depression, in general, have decreased levels of inositol in their cerebrospinal fluid.[18][19] A double-blind, placebo-controlled study of depressed patients showed that a high dose of inositol (12 grams daily) resulted in significant improvement of symptoms, with no changes noted in liver, kidney, or hematological function.[18][19][21] A meta-analysis of randomized trials of inositol for depression was not able to determine if inositol is of benefit.[23]
Older research suggests that lithium functions primarily by decreasing myo-inositol concentrations in bipolar patients; however the conclusions of this research are unsupported and have been questioned.[24][25] Other studies suggest that lithium treatment may further inhibit the enzyme inositol monophosphatase, leading to higher intracellular levels of inositol triphosphate,[26] an effect that was enhanced further by administration of an inositol triphosphate reuptake inhibitor.
Other conditions
D-chiro-inositol (DCI) has been found in two double-blind studies to be an effective treatment for many of the clinical hallmarks of polycystic ovary syndrome (PCOS), including insulin resistance, hyperandrogenism, and oligo-amenorrhea;[27][28] the impetuses for these studies were the observed defects in DCI metabolism in PCOS and the implication of DCI in insulin signal transduction.[7][29] Another small, placebo-controlled study has demonstrated that myo-inositol supplementation improves features of dysmetabolic syndrome in post-menopausal women, including triglycerides, HDL cholesterol, and diastolic blood pressure.[30]
Animal studies suggest inositol reduces the severity of the osmotic demyelination syndrome if given prior to rapid correction of chronic hyponatraemia.[31] Further study is required prior to its application in humans for this indication.
Studies from in vitro experiments, animal studies, and limited clinical experiences, claim that inositol may be used effectively against some types of cancer, in particular, when used in combination with phytic acid.[32]
Common use as a "cutting" agent
Inositol has been used as an adulterant (or cutting agent) in many illegal drugs, such as cocaine, methamphetamine, and sometimes heroin.[citation needed] It is presumed that this use is connected with the substance's solubility and near-lack of taste (which is easily hidden by that of the drugs themselves).
Nutritional sources
Myo-inositol is naturally present in a variety of foods, although tables of this do not always distinguish between the bioavailable lecithin form, and the non-available phytate form in grains.[33] According to research, foods containing the highest concentrations of myo-inositol (including its compounds) include fruits, beans, grains and nuts.[33] Beans and grains, however, as seeds contain large amounts of inositol as phytate.
See also
- Allo-inositol
- Cis-inositol
- D-chiro-inositol
- Epi-inositol
- L-chiro-inositol
- Muco-inositol
- Neo-inositol
- Scyllo-inositol
- Inositol 1-methyltransferase
- Inositol 3-methyltransferase
- Inositol 4-methyltransferase
- Inositol nicotinate
- Inositol phosphate
- Inositol trisphosphate
- Inositol pentakisphosphate
- Inositol hexaphosphate
- Inositol triphosphate receptor
- Inositol hexanicotinate
References
- ^ Merck Index, 11th Edition, 4883.
- ^ Synonyms in PubChem
- ^ Synonyms in Commonchemistry.org
- ^ Clements RS Jr, Darnell B (1980). "Myo-inositol content of common foods: development of a high-myo-inositol diet" (PDF). American Journal of Clinical Nutrition 33 (9): 1954–1967. PMID 7416064. http://www.ajcn.org/content/33/9/1954.long.
- ^ Reynolds, James E. F. (January 1, 1993). Martindale: The Extra Pharmacopoeia. 30. Pennsylvania: Rittenhouse Book Distributors. pp. 1379. ISBN 0853693005. "An isomer of glucose that has traditionally been considered to be a B vitamin although it has an uncertain status as a vitamin and a deficiency syndrome has not been identified in man"
- ^ The Chemical and Bio-physical properties of Phosphatidylinositol phosphates, Thesis for M.Res.. Imperial College London. 2006.
- ^ a b Larner J (2002). "D-chiro-inositol--its functional role in insulin action and its deficit in insulin resistance". Int J Exp Diabetes Res 3 (1): 47–60. doi:10.1080/15604280212528. PMC 2478565. PMID 11900279. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2478565.
- ^ Gerasimenko, Julia V; et al; “Bile Acids Induce Ca2+ Release from Both the Endoplasmic Reticulum and Acidic Intracellular Calcium Stores through Activation of Inositol Trisphosphate Receptors and Ryanodine Receptors”; Journal of Biological Chemistry; December 29, 2006; Volume 281: Pp 40154-40163.
- ^ Kukuljan M, Vergara L, Stojilkovic SS (February 1997). "Modulation of the kinetics of inositol 1,4,5-trisphosphate-induced [Ca2+i oscillations by calcium entry in pituitary gonadotrophs"]. Biophysical Journal 72 (2 Pt 1): 698–707. Bibcode 1997BpJ....72..698K. doi:10.1016/S0006-3495(97)78706-X. PMC 1185595. PMID 9017197. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1185595.
- ^ Rapiejko PJ, Northup JK, Evans T, Brown JE, Malbon CC (November 1986). "G-proteins of fat-cells. Role in hormonal regulation of intracellular inositol 1,4,5-trisphosphate". The Biochemical Journal 240 (1): 35–40. PMC 1147372. PMID 3103610. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1147372.
- ^ Shen, X.; Xiao, H; Ranallo, R; Wu, WH; Wu, C (2003). "Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates". Science 299 (5603): 112–4. doi:10.1126/science.1078068. PMID 12434013.
- ^ Steger, D. J.; Haswell, ES; Miller, AL; Wente, SR; O'Shea, EK (2003). "Regulation of chromatin remodelling by inositol polyphosphates". Science 299 (5603): 114–6. doi:10.1126/science.1078062. PMC 1458531. PMID 12434012. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1458531.
- ^ Phytic acid
- ^ Hurrell RF (September 2003). "Influence of vegetable protein sources on trace element and mineral bioavailability". The Journal of Nutrition 133 (9): 2973S–7S. PMID 12949395. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=12949395.
- ^ Committee on Food Protection, Food and Nutrition Board, National Research Council (1973). "Phytates". Toxicants Occurring Naturally in Foods. National Academy of Sciences. pp. 363–371. ISBN 9780309021173. http://books.google.com/?id=lIsrAAAAYAAJ&pg=PA363.
- ^ Laurence, William L. "Corn by-product yields explosive", The New York Times. April 17, 1936. Page 7.
- ^ Ledgard, Jared. The Preparatory Manual of Explosives, 2007. p. 366.
- ^ a b c Nick, Gina L. (2004). "Inositol as a treatment for psychiatric disorders: a scientific evaluation of its clinical effectiveness". (indirect through findarticles.com) Townsend Letter for Doctors and Patients (October). http://findarticles.com/p/articles/mi_m0ISW/is_255/ai_n6211958. Retrieved 2008-05-24.
- ^ a b c Nick, Gina L. (2004). "Inositol as a treatment for psychiatric disorders". (direct) Townsend Letter; the Examiner of Alternative Medicine (October). http://www.townsendletter.com/Oct2004/Oct2004.htm.
- ^ a b Palatnik A, Frolov K, Fux M, Benjamin J (2001). "Double-blind, controlled, crossover trial of inositol versus fluvoxamine for the treatment of panic disorder". Journal of Clinical Psychopharmacology 21 (3): 335–339. doi:10.1097/00004714-200106000-00014. PMID 11386498.
- ^ a b Levine J, Barak Y, Gonzalves M, Szor H, Elizur A, Kofman O, Belmaker RH. (1995). "Double-blind, controlled trial of inositol treatment of depression". American Journal of Psychiatry 152 (5): 792–794. PMID 7726322.
- ^ Fux M, Levine J, Aviv A, Belmaker RH (1996). "Inositol treatment of obsessive-compulsive disorder". American Journal of Psychiatry 153 (9): 1219–21. PMID 8780431.
- ^ Taylor MJ, Wilder H, Bhagwagar Z, Geddes J (2004). Taylor, Matthew J. ed. "Inositol for depressive disorders". Cochrane Database Syst Rev (2): CD004049. doi:10.1002/14651858.CD004049.pub2. PMID 15106232.
- ^ Silverstone, P. H.; McGrath, B. M.; Kim, H. (2005). "Bipolar disorder and myo-inositol: A review of the magnetic resonance spectroscopy findings". Bipolar Disorders 7 (1): 1–10. doi:10.1111/j.1399-5618.2004.00174.x. PMID 15654927.
- ^ Harwood, AJ (2005). "Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited". Molecular Psychiatry 10: 117–126. doi:10.1038/sj.mp.4001618. http://www.nature.com/mp/journal/v10/n1/full/4001618a.html.
- ^ Einat H, Kofman O, Itkin O, Lewitan RJ, Belmaker RH (1998). "Augmentation of lithium's behavioral effect by inositol uptake inhibitors". J Neural Transm 105 (1): 31–8. doi:10.1007/s007020050035. PMID 9588758. http://link.springer.de/link/service/journals/00702/bibs/8105001/81050031.htm.
- ^ Nestler J E, Jakubowicz D J, Reamer P, Gunn R D, Allan G (1999). "Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome". N Engl J Med 340 (17): 1314–1320. doi:10.1056/NEJM199904293401703. PMID 10219066.
- ^ Iuorno M J, Jakubowicz D J, Baillargeon J P, Dillon P, Gunn R D, Allan G, Nestler J E (2002). "Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome". Endocr Pract 8 (6): 417–423. PMID 15251831.
- ^ Nestler J E, Jakubowicz D J, Iuorno M J (2000). "Role of inositolphosphoglycan mediators of insulin action in the polycystic ovary syndrome". J Pediatr Endocrinol Metab 13 Suppl 5: 1295–1298. PMID 11117673.
- ^ Giordano D, Corrado F, Santamaria A, Quattrone S, Pintaudi B, DiBenedetto A, D’Anna R (2011). "Effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome: a perspective, randomized, placebo-controlled study". Menopause: The Journal of The North American Menopause Society 18 (1): 102–104. doi:10.1097/gme.0b013e3181e8e1b1.
- ^ Silver SM, Schroeder BM, Sterns RH, Rojiani AM (2006). "Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats". J Neuropathol Exp Neurol 65 (1): 37–44. doi:10.1097/01.jnen.0000195938.02292.39. PMID 16410747.
- ^ Vucenik, I; Shamsuddin, AM (2003). "Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic.". The Journal of nutrition 133 (11 Suppl 1): 3778S–3784S. PMID 14608114.
- ^ a b Clements, Rex; Betty Darnell (1980). "Myo-inositol content of common foods: development of a high-myo-inositol diet". American Journal of Clinical Nutrition 33 (9): 1954–1967. PMID 7416064. http://www.ajcn.org/cgi/reprint/33/9/1954.pdf. Retrieved 2009-05-18.
External links
- Cancer Inhibition by Inositol Hexaphosphate (IP6) and Inositol: From Laboratory to Clinic (scientific publication)
- Myo-inositol Content of Various Foods
- U.S. National Library of Medicine: Drug Information Portal - Inositol
- 'Anxiety, Depression, Antibiotics and Inositol'
Anxiety disorder: Obsessive–compulsive disorder (F42, 300.3) History Yale–Brown Obsessive Compulsive ScaleBiology NeuroanatomyReceptorsSymptoms Obsessions (associative, diagnostic, injurious, scrupulous, pathogenic, sexual) · Compulsions (impulses, rituals, tics) · Thought suppression (avoidance) · Hoarding (animals, books, possessions)Treatment InositolMu opioidergicsNMDA glutamatergicsNK-1 tachykininergicsOtherBehavioralOrganizations Notable people Edna B. Foa · Stanley Rachman · Adam S. Radomsky · Jeffrey M. Schwartz · Susan Swedo · Jeff Bell · Emily ColasPopular culture LiteratureFictionalNonfictionMediaRelated Obsessive–compulsive personality disorder · Obsessional jealousy · Purely Obsessional OCD · Social anxiety disorder · Tourette syndromeGlycerol backbone
(Glycerophospholipids/
Phosphoglycerides)Phosphatidyl-: -ethanolamine/cephalin (PE) · -choline/lechithin (PC) · -serine (PS) · -glycerol (PG) · -inositol (PI) (glyco- (GPI))
Phosphoinositides: PIP (PI(3)P, PI(4)P, PI(5)P) · PIP2 (PI(3,4)P2, PI(3,5)P2, PI(4,5)P2) · PIP3
Ether lipids: Plasmalogen (Platelet-activating factor)Sphingosine backbone Metabolites biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Inositol
- Xanthine oxidase inhibitors
- Chemopreventive agents
- Biology of bipolar disorder
- Treatment of obsessive–compulsive disorder
- Biology of obsessive–compulsive disorder
Wikimedia Foundation. 2010.
