- Nitrocellulose
-
Nitrocellulose[1] 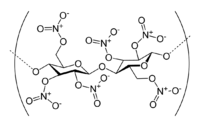
 Other namesCellulose nitrate; Flash paper; Gun cotton; Collodion; Pyroxylin
Other namesCellulose nitrate; Flash paper; Gun cotton; Collodion; PyroxylinIdentifiers CAS number 9004-70-0 
Properties Molar mass Variable Appearance Yellowish white cotton-like filaments Melting point 160–170 °C (ignites)
Hazards Flash point 4.4 °C LD50 10 mg/kg (mouse, IV)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitrocellulose (also: cellulose nitrate, flash paper) is a highly flammable compound formed by nitrating cellulose through exposure to nitric acid or another powerful nitrating agent. When used as a propellant or low-order explosive, it is also known as guncotton. Nitrocellulose plasticized by camphor was used by Kodak, and other suppliers, from the late 1880s as a film base in photograph, X-ray films and motion picture films; and was known as nitrate film. After numerous fires caused by unstable nitrate films, safety film started to be used from the 1930s in the case of X-ray stock and from 1948 for motion picture film.
Contents
Guncotton
Henri Braconnot discovered in 1832 that nitric acid, when combined with starch or wood fibers, would produce a lightweight combustible explosive material, which he named xyloïdine. A few years later in 1838 another French chemist Théophile-Jules Pelouze (teacher of Ascanio Sobrero and Alfred Nobel) treated paper and cardboard in the same way. He obtained a similar material he called nitramidine. Both of these substances were highly unstable, and were not practical explosives.
However, around 1846 Christian Friedrich Schönbein, a German-Swiss chemist, discovered a more practical solution. As he was working in the kitchen of his home in Basle, he spilled a bottle of concentrated nitric acid on the kitchen table. He reached for the nearest cloth, a cotton apron, and wiped it up. He hung the apron on the stove door to dry, and, as soon as it was dry, there was a flash as the apron exploded. His preparation method was the first to be widely imitated — one part of fine cotton wool to be immersed in fifteen parts of an equal blend of sulfuric and nitric acids. After two minutes, the cotton was removed and washed in cold water to set the esterification level and remove all acid residue. It was then slowly dried at a temperature below 100 °F (about 38° C). Schönbein collaborated with the Frankfurt professor Rudolf Christian Böttger, who had discovered the process independently in the same year. By coincidence, a third chemist, the Brunswick professor F. J. Otto had also produced guncotton in 1846 and was the first to publish the process, much to the disappointment of Schönbein and Böttger.[2]
The process uses the nitric acid to convert the cellulose into cellulose nitrate and water:
- 3HNO3+ C6H10O5 → C6H7(NO2)3O5 + 3H2O
The sulfuric acid is present as a catalyst to produce the nitronium ion, NO2+. The reaction is first order and proceeds by electrophilic substitution at the C-OH centers of the cellulose.[3]
The power of guncotton made it suitable for blasting. As a projectile driver, it has around six times the gas generation of an equal volume of black powder and produces less smoke and less heating. However, the sensitivity of the material during production led the British, Prussians and French to discontinue manufacture within a year.
Jules Verne viewed the development of guncotton with optimism. He referred to the substance several times in his novels. His adventurers carried firearms employing this substance. The most noteworthy reference is in his From the Earth to the Moon, in which guncotton was used to launch a projectile into space.
Further research indicated that the key was the very careful preparation of the cotton: Unless it was very well cleaned and dried, it was likely to explode spontaneously. The British, led by Frederick Augustus Abel, also developed a much lengthier manufacturing process at the Waltham Abbey Royal Gunpowder Mills, patented in 1865, with the washing and drying times each extended to 48 hours and repeated eight times over. The acid mixture was also changed to two parts sulfuric acid to one part nitric.
Guncotton remained of limited use. For firearms, a more stable and slower burning mixture was needed. Guncotton-like preparations were eventually prepared for this role, known at the time as smokeless powder. The first practical smokeless powder made from nitrocellulose, for firearms and artillery ammunition, was invented by French chemist Paul Vieille in 1884.
Guncotton, dissolved at approximately 25% in acetone, forms a lacquer used in preliminary stages of wood finishing to develop a hard finish with a deep lustre. It is normally the first coat applied, sanded and followed by other coatings that bond to it.
Nitrate film
Nitrocellulose was used as the first flexible film base, beginning with Eastman Kodak products in August, 1889. Camphor is used as a plasticizer for nitrocellulose film, often called nitrate film. It was used until 1933 for X-ray films (where its flammability hazard was most acute) and for motion picture film until 1951. It was replaced by safety film with an acetate base.
The use of nitrocellulose film for motion pictures led to the requirement for fireproof projection rooms with wall coverings made of asbestos. The US Navy shot a training film for projectionists that included footage of a controlled ignition of a reel of nitrate film, which continued to burn when fully submerged in water. Unlike many other flammable materials, nitrocellulose does not need air to keep burning as the reaction produces oxygen. Once burning, it is extremely difficult to extinguish. Immersing burning film in water may not extinguish it, and could actually increase the amount of smoke produced.[4][5] Owing to public safety precautions, the London Underground forbade transport of movies on its system until well past the introduction of safety film.
Cinema fires caused by ignition of nitrocellulose film stock were the cause of the 1926 Dromcolliher cinema tragedy in County Limerick in which 48 people died and the 1929 Glen Cinema Disaster which killed 69 children. Today, nitrate film projection is normally highly regulated and requires extensive precautionary measures including extra projectionist health and safety training. Projectors certified to run nitrate films have many precautions, among them the chambering of the feed and takeup reels in thick metal covers with small slits to allow the film to run through. The projector is modified to accommodate several fire extinguishers with nozzles aimed at the film gate. The extinguishers automatically trigger if a piece of flammable fabric placed near the gate starts to burn. While this triggering would likely damage or destroy a significant portion of the projection components, it would prevent a fire which could cause far greater damage. Projection rooms may be required to have automatic metal covers for the projection windows, preventing the spread of fire to the auditorium.
It was found that nitrocellulose gradually decomposes, releasing nitric acid and further catalyzing the decomposition (eventually into a flammable powder or goo). Decades later, storage at low temperatures was discovered as a means of delaying these reactions indefinitely. It is thought that the great majority of films produced during the early twentieth century were lost either through this accelerating, self-catalyzed disintegration or through studio warehouse fires. Salvaging old films is a major problem for film archivists (see film preservation).
Nitrocellulose film base manufactured by Kodak can be identified by the presence of the word Nitrate in dark letters between the perforations. Acetate film manufactured during the era when nitrate films were still in use was marked Safety or Safety Film between the perforations in dark letters. Film stocks in the non-standard gauges, 8 mm or 16 mm, were not manufactured with a nitrate base on any significant scale in the west, though rumours persist of 16mm nitrate having been produced in the former Soviet Union and/or China.[6]
Replacement filmstocks
Nitrate dominated the market for professional use 35mm motion picture film from the industry's origins to the early 1950s. While cellulose acetate-based so-called 'safety film', notably cellulose diacetate and cellulose acetate propionate, was produced in the gauge for small-scale use in niche applications (e.g. printing advertisements and other short films to enable them to be sent through the post without the need for fire safety precautions), the early generations of safety film base had two major disadvantages relative to nitrate: it was a lot more expensive to manufacture, and a lot less durable in repeated projection. The cost of the safety precautions associated with the use of nitrate was significantly lower than the cost of using any of the safety bases available before 1948. These drawbacks were eventually overcome with the launch of cellulose triacetate base film by Eastman Kodak in 1948.[7] Cellulose triacetate superseded nitrate as the film industry's mainstay base very quickly: Kodak announced the discontinuation of nitrate manufacture in February 1950.
The crucial advantage cellulose triaecetate had over nitrate was that it was no more of a fire risk than paper (the stock is often erroneously referred to as 'non-flam': this is not true - it is combustible, but not in as volatile or as dangerous a way as nitrate), while it almost matched the cost and durability of nitrate. It remained in almost exclusive use in all film gauges until the 1980s, when polyester, or PET film, began to supersede it for intermediate and release printing.[8]
Polyester is much more resistant to polymer degradation than either nitrate or triacetate. Although triacetate does not decompose in as dangerous a way as nitrate does, it is still subject to a process known as deacetylation, often nicknamed 'vinegar syndrome' (due to the acetic acid smell of decomposing film) by archivists, which causes the film to shrink, deform, become brittle and eventually unusable. Polyethylene terephthalate, like Cellulose Mononitrate, is less prone to stretching than other available plastics. By the late 1990s polyester had almost entirely superseded triacetate for the production of intermediate elements and release prints.
Triacetate remains in use for most camera negative stocks because it can be 'invisibly' spliced using solvents during negative assembly, while polyester film can only be spliced using adhesive tape patches or ultrasonically, both of which will leave visible marks in the frame area. Also triacetate film will break under tension, whereas polyester will not, reducing the risk of very serious damage to expensive camera mechanisms in the event of a film jam. This later point applies to projectors as well, of course. There were many opposed to the use of polyester for release prints for precisely this reason, and because ultrasonic splicers are very expensive items, beyond the budgets of many smaller theaters. However in practice this has not proved to be anything like such a problem as was feared. Rather, with the increased use of automated long-play systems in cinemas, the greater strength of polyester has been a significant advantage in lessening the risk of a film performance being interrupted by a film break.
Production
Guncotton
In general, cotton was used as the cellulose base, and is added to concentrated sulfuric acid and 70% nitric acid cooled to 0oC to give cellulose trinitrate (or guncotton).
While guncotton is dangerous to store, its risks can be reduced by storing it wet or in oil.
Nitrate film
Cellulose is treated with sulfuric acid and potassium nitrate to give cellulose mononitrate. This was used commercially as Celluloid, a highly flammable plastic used in the first half of the 20th Century for lacquers and photographic film.[9]
Uses
 An M13 rocket for the Katyusha launcher on display in the Musée de l'Armée. Its solid-fuel rocket motor was prepared from nitrocellulose.
An M13 rocket for the Katyusha launcher on display in the Musée de l'Armée. Its solid-fuel rocket motor was prepared from nitrocellulose.
- A nitrocellulose slide, nitrocellulose membrane or nitrocellulose paper is a sticky membrane used for immobilizing nucleic acids in Southern blots and northern blots. It is also used for immobilization of proteins in Western blots and Atomic Force Microscopy[10] for its non-specific affinity for amino acids. Nitrocellulose is widely used as support in diagnostic tests where antigen-antibody binding occur, e.g., pregnancy tests, U-Albumin tests and CRP. Glycine and chloride ions make protein transfer more efficient.
- When the solution is dissolved in ether, alcohol or other organic solvents it produces collodion, discovered in 1846 and introduced as a wound dressing during the Crimean War. It is still in use today in topical skin applications, such as liquid skin and in the application of salicylic acid, the active ingredient in Compound W wart remover.
- In 1851, Frederick Scott Archer invented the Wet Collodion Process as a replacement for albumen in early photographic emulsions, binding light-sensitive silver halides to a glass plate.[11]
- Magician's flash paper, sheets of paper or cloth made from nitrocellulose, which burn almost instantly with a bright flash leaving no ash.
- Radon tests for alpha track etches.
- Nitrocellulose lacquer was used as a finish on guitars and saxophones for most of the 20th century and is still used on some current applications. Manufactured by (among others) DuPont, the paint was also used on automobiles sharing the same color codes as many guitars including Fender and Gibson brands,[12] although it fell out of favor for a number of reasons: pollution, and the way the lacquer yellows and cracks over time.
- Nitrocellulose lacquer is also used as an aircraft dope, painted onto fabric-covered aircraft to tauten and provide protection to the material.
- As a medium for cryptographic one-time pads, thus making the disposal of the pad complete, secure, and efficient.
- Nitrocellulose lacquer is spin-coated onto aluminum or glass discs, then a groove is cut with a lathe, to make one-off phonograph records, used as masters for pressing or for play in dance clubs. They are referred to as acetate discs.
- Depending on the manufacturing process, nitrocellulose is esterified to varying degrees. Table tennis balls, guitar picks and some photographic films have a fairly low esterification level and burn comparatively slowly with some charred residue. See celluloid.
Because of its explosive nature, not all applications of nitrocellulose were successful. In 1869, with elephants having been poached to near extinction, the billiards industry offered a $10,000 prize to whoever came up with the best replacement for ivory billiard balls. John Wesley Hyatt created the winning replacement which he coated with a new material he discovered called camphored nitrocellulose—the first thermoplastic, better known as celluloid. The invention enjoyed a brief popularity, but the Hyatt balls were extremely flammable, and sometimes portions of the outer shell would explode upon impact. An owner of a billiard saloon in Colorado wrote to Hyatt about the explosive tendencies, saying that he did not mind very much personally but for the fact that every man in his saloon immediately pulled a gun at the sound.[13][14] The process used by Hyatt to manufacture the billiard balls, (US Patent 239,792, 1881) involved placing the mass of nitrocellulose in a rubber bag, which was then placed in a cylinder of liquid and heated. Pressure was applied to the liquid in the cylinder, which resulted in a uniform compression on the nitrocellulose mass, compressing it into a uniform sphere as the heat vaporized the solvents. The ball was then cooled and turned to make a uniform sphere. In light of the explosive results, this process was called the "Hyatt Gun Method".[15]
See also
- Cellulose
- Smokeless powder
- Cordite
- Nitroglycerine
- Nitrostarch
- Potassium nitrate
References
- ^ Merck Index, 11th Edition, 8022.
- ^ Itzehoer Wochenblatt, 29 October 1846, columns 1626 f.
- ^ Urbanski, Tadeusz, Chemistry and Technology of Explosives, Pergamon Press, Oxford, 1965, Vol 1, pp 20–21.
- ^ Health and Safety Executive leaflet/cellulose.pdf
- ^[dead link]Interesting discussion on NC films.
- ^ David Cleveland, 'Don't Try This at Home: Some Thoughts on Nitrate Film, With Particular Rerefence to Home Movie Systems' in Roger Smither and Catherine Surowiec (eds.), This Film is Dangerous: A Celebration of Nitrate Film, Brussels, FIAF (2002), ISBN 978-2960029604, p. 196
- ^ Charles Fordyce et al, 'Improved Safety Motion Picture Film Support', Journal of the SMPE, vol. 51 (October 1948), pp. 331-350
- ^ George J. van Schil, 'The Use of Polyester Film Base in the Motion Picture Industry', SMPTE Journal, vol. 89, no. 2 (February 1980), pp. 106-110.
- ^ http://fliiby.com/file/208138/a7bake2p2k.html
- ^ L. Kreplak et al. Atomic Force Microscopy of Mammalian Urothelial Surface. Journal of molecular biology. Volume 374, Issue 2, 23 November 2007, Pages 365-373
- ^ Dr. R Leggat, A History of Photography: The Collodion Process
- ^ "What is "stand damage"?". http://www.gibson.com/en-us/Support/FAQs/#.
- ^ Connections, James Burke, Volume 9, "Countdown", 29:00 – 31:45, 1978
- ^ United States. National Resources Committee (1941). RESEARCH—A NATIONAL RESOURCE. UNITED STATES GOVERNMENT PRINTING OFFICE. p. 29.
- ^ Edward Chauncey Worden (1911). Nitrocellulose Industry, Volume 2. D. Van Nostrand Company. pp. 726–727.
External links
Wikimedia Foundation. 2010.



