- Cyamemazine
-
Cyamemazine 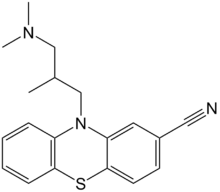
Systematic (IUPAC) name 10-(3-dimethylamino-2-methyl-propyl)phenothiazine-2-carbonitrile Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status Rx-Only Routes Oral, IM, IV Pharmacokinetic data Bioavailability 10-70% Metabolism Hepatic Half-life 10 hours Excretion Urine Identifiers CAS number 3546-03-0 ATC code N05AA06 PubChem CID 62865 IUPHAR ligand 84 ChemSpider 56597 
UNII A2JGV5CNU4 
KEGG D07307 
Chemical data Formula C19H21N3S Mol. mass 323.46 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Cyamemazine (Tercian), also known as cyamepromazine, is a typical antipsychotic drug of the phenothiazine class which was introduced by Theraplix in France in 1972 and later in Portugal as well.[1][2][3][4] It is used for the treatment of schizophrenia and, especially, for psychosis-associated anxiety, due to its unique anxiolytic efficacy.[5][6]
Cyamemazine differs from other phenothiazine neuroleptics in that aside from the usual profile of dopamine, α1-adrenergic, H1, and mACh receptor antagonism,[7] it additionally produces potent blockade of several serotonin receptors, including 5-HT2A, 5-HT2C, and 5-HT7.[7][8][9][10] These actions have been implicated in cyamemazine's anxiolytic effects (5-HT2C) and lack of extrapyramidal side effects (5-HT2A),[7][8] and despite being classified as a typical, it actually behaves like an atypical antipsychotic.[11]
See also
References
- ^ Index nominum, international drug ... - Google Books. http://books.google.com/books?id=5GpcTQD_L2oC&lpg=PA280&dq=cyamemazine%20tercian&as_brr=3&pg=PA280#v=onepage&q=&f=false.
- ^ David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. p. 534. ISBN 0-412-46630-9. http://books.google.com/books?id=DeX7jgInYFMC&lpg=RA1-PA534&as_brr=3&pg=RA1-PA534#v=onepage&q=&f=false.
- ^ Pharmaceutical manufacturing ... - Google Books. http://books.google.com/books?id=X2EyLsG4bcUC&lpg=PA397&dq=cyamemazine%20introduced&as_brr=3&pg=PA397#v=onepage&q=&f=false.
- ^ Bret P, Bret MC, Queuille E (April 2009). "[Prescribing patterns of antipsychotics in 13 French psychiatric hospitals"] (in French). L'Encéphale 35 (2): 129–38. doi:10.1016/j.encep.2008.03.007. PMID 19393381. http://www.masson.fr/masson/S0013-7006(08)00103-6.
- ^ "Stahl's Essential Psychopharmacology - Cambridge University Press". http://stahlonline.cambridge.org/prescribers_drug.jsf?page=0521683505c20_p115-120.html.therapeutics&name=Cyamemazine.
- ^ Bourin M, Nic Dhonnchadha BA, Claude Colombel M, Dib M, Hascoët M (September 2001). "Cyamemazine as an anxiolytic drug on the elevated plus maze and light/dark paradigm in mice". Behavioural Brain Research 124 (1): 87–95. doi:10.1016/S0166-4328(01)00238-8. PMID 11423169. http://linkinghub.elsevier.com/retrieve/pii/S0166432801002388.
- ^ a b c Hameg A, Bayle F, Nuss P, Dupuis P, Garay RP, Dib M (February 2003). "Affinity of cyamemazine, an anxiolytic antipsychotic drug, for human recombinant dopamine vs. serotonin receptor subtypes". Biochemical Pharmacology 65 (3): 435–40. doi:10.1016/S0006-2952(02)01515-0. PMID 12527336. http://linkinghub.elsevier.com/retrieve/pii/S0006295202015150.
- ^ a b Alvarez-Guerra M, d'Alché-Birée F, Wolf WA, Vargas F, Dib M, Garay RP (January 2000). "5-HT3- and 5-HT2C-antagonist properties of cyamemazine: significance for its clinical anxiolytic activity". Psychopharmacology 147 (4): 412–7. doi:10.1007/s002130050010. PMID 10672635. http://link.springer.de/link/service/journals/00213/bibs/0147004/01470412.htm.
- ^ Alvarez-Guerra M, Hameg A, Bayle F, Dib M, Garay RP (November 2002). "5-HT2A receptor antagonist properties of cyamemazine in rat and guinea pig smooth muscle". European Journal of Pharmacology 454 (2-3): 235–9. doi:10.1016/S0014-2999(02)02489-5. PMID 12421652. http://linkinghub.elsevier.com/retrieve/pii/S0014299902024895.
- ^ Benyamina A, Arbus C, Nuss P, Garay RP, Neliat G, Hameg A (January 2008). "Affinity of cyamemazine metabolites for serotonin, histamine and dopamine receptor subtypes". European Journal of Pharmacology 578 (2-3): 142–7. doi:10.1016/j.ejphar.2007.09.025. PMID 17936750. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(07)01053-9.
- ^ Peinado J, Hameg A, Garay RP, Bayle F, Nuss P, Dib M (February 2003). "Reduction of extracellular dopamine and metabolite concentrations in rat striatum by low doses of acute cyamemazine". Naunyn-Schmiedeberg's Archives of Pharmacology 367 (2): 134–9. doi:10.1007/s00210-002-0665-4. PMID 12595954.
Adrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Teniloxazine • Tramadol • ZiprasidoneReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • TuaminoheptaneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugs Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeDopaminergics Receptor ligands AgonistsAdamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635AntagonistsTypical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Cariprazine • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • YohimbineReuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugs Histaminergics Receptor
ligandsAgonists: 2-Pyridylethylamine • Betahistine • Histamine • HTMT • UR-AK49
Antagonists: 1st generation: 4-Methyldiphenhydramine • Alimemazine • Antazoline • Azatadine • Bamipine • Benzatropine (Benztropine) • Bepotastine • Bromazine • Brompheniramine • Buclizine • Captodiame • Carbinoxamine • Chlorcyclizine • Chloropyramine • Chlorothen • Chlorphenamine • Chlorphenoxamine • Cinnarizine • Clemastine • Clobenzepam • Clocinizine • Cyclizine • Cyproheptadine • Dacemazine • Deptropine • Dexbrompheniramine • Dexchlorpheniramine • Dimenhydrinate • Dimetindene • Diphenhydramine • Diphenylpyraline • Doxylamine • Embramine • Etybenzatropine (Ethylbenztropine) • Etymemazine • Histapyrrodine • Homochlorcyclizine • Hydroxyethylpromethazine • Hydroxyzine • Isopromethazine • Isothipendyl • Meclozine • Mepyramine (Pyrilamine) • Mequitazine • Methafurylene • Methapyrilene • Methdilazine • Moxastine • Niaprazine • Orphenadrine • Oxatomide • Oxomemazine • Phenindamine • Pheniramine • Phenyltoloxamine • Pimethixene • Piperoxan • Promethazine • Propiomazine • Pyrrobutamine • Talastine • Thenalidine • Thenyldiamine • Thiazinamium • Thonzylamine • Tolpropamine • Tripelennamine • Triprolidine; 2nd generation: Acrivastine • Astemizole • Azelastine • Cetirizine • Clemizole • Clobenztropine • Ebastine • Emedastine • Epinastine • Ketotifen • Latrepirdine • Levocabastine • Loratadine • Mebhydrolin • Mizolastine • Olopatadine • Rupatadine • Setastine • Terfenadine; "3rd generation": Desloratadine • Fexofenadine • Levocetirizine; Miscellaneous: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc) • Serotonin antagonist and reuptake inhibitors (Trazodone, Nefazodone) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc) • Atypical antipsychotics (Clozapine, Olanzapine, Quetiapine, etc)Agonists: Amthamine • Betazole • Dimaprit • Histamine • HTMT • Impromidine • UR-AK49
Antagonists: Burimamide • Cimetidine • Ebrotidine • Famotidine • Lafutidine • Lavoltidine/Loxtidine • Lupitidine • Metiamide • Niperotidine • Nizatidine • Oxmetidine • Ranitidine • RoxatidineAgonists: α-Methylhistamine • Cipralisant • Histamine • Imetit • Immepip • Immethridine • Methimepip • Proxyfan
Antagonists: A-349,821 • A-423,579 • ABT-239 • Betahistine • Burimamide • Ciproxifan • Clobenpropit • Conessine • GSK-189,254 • Impentamine • Iodophenpropit • JNJ-5,207,852 • MK-0249 • NNC-38-1,049 • PF-03654746 • Pitolisant • SCH-79,687 • Thioperamide • VUF-5,681Agonists: 4-Methylhistamine • Histamine • VUF-8,430
Antagonists: JNJ-7,777,120 • Thioperamide • VUF-6,002Reuptake
inhibitorsVMAT inhibitorsEnzyme
inhibitorsHDC inhibitorsCatechin • Meciadanol • Naringenin • TritoqualineHNMT inhibitorsDAO inhibitorsAminoguanidineOthers L-HistidineSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersTricyclics Classes Acridine • Anthracene • Dibenzazepine • Dibenzocycloheptene • Dibenzodiazepine • Dibenzothiazepine • Dibenzothiepin • Dibenzoxazepine • Dibenzoxepin • Phenothiazine • Pyridazinobenzoxazine • Pyridinobenzodiazepine • ThioxantheneAntidepressants 7-OH-Amoxapine • Amezepine • Amineptine • Amitriptyline • Amitriptylinoxide • Amoxapine • Aptazapine • Azepindole • Azipramine • Butriptyline • Cianopramine • Ciclazindol • Ciclopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Esmirtazapine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Loxapine • Maprotiline • Mariptiline • Mazindol • Melitracen • Metapramine • Mezepine • Mianserin • Mirtazapine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Oxaprotiline • Pipofezine • Pirandamine • Propizepine • Protriptyline • Quinupramine • Setiptiline/Teciptiline • Tandamine • Tampramine • Tianeptine • Tienopramine • TrimipramineAntihistamines Alimemazine • Azatadine • Clobenzepam • Cyproheptadine • Dacemazine • Deptropine • Desloratadine • Epinastine • Etymemazine • Hydroxyethylpromethazine • Isopromethazine • Isothipendyl • Ketotifen • Latrepirdine • Loratadine • Mebhydrolin • Mequitazine • Methdilazine • Olopatadine • Oxomemazine • Phenindamine • Pimethixene • Promethazine • Propiomazine • Rupatadine • ThiazinamiumAntipsychotics Acetophenazine • Amoxapine • Asenapine • Butaclamol • Butaperazine • Carphenazine • Carpipramine • Chlorpromazine • Chlorprothixene • Ciclindole • Clocapramine • Clomacran • Clotiapine • Clozapine • Flucindole • Fluotracen • Flupentixol • Fluphenazine • Gevotroline • Homopipramol • Levomepromazine/Methotrimeprazine • Loxapine • Maroxepin • Mesoridazine • Metitepine/Methiothepin • Metoxepin • Mosapramine • Naranol • Olanzapine • Perazine • Perphenazine • Periciazine • Piperacetazine • Pipotiazine • Piquindone • Prochlorperazine • Promazine • Prothipendyl • Quetiapine • Sulforidazine • Thiethylperazine • Thiopropazate • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Zotepine • ZuclopenthixolOthers Atiprosin • Carbamazepine • Carvedilol • Cyclobenzaprine • Licarbazepine • Methylene Blue • Monatepil • Oxcarbazepine • Oxitriptyline • Pirenzepine • Pirolate • Pitrazepin • Pizotifen • Profenamine
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it.
