- Measuring instrument
-
 Captain Nemo and Professor Aronnax contemplating measuring instruments in Twenty Thousand Leagues Under the Sea
Captain Nemo and Professor Aronnax contemplating measuring instruments in Twenty Thousand Leagues Under the Sea A Love Meter at a Framingham, Massachusetts Rest Stop.
A Love Meter at a Framingham, Massachusetts Rest Stop.
In the physical sciences, quality assurance, and engineering, measurement is the activity of obtaining and comparing physical quantities of real-world objects and events. Established standard objects and events are used as units, and the process of measurement gives a number relating the item under study and the referenced unit of measurement. Measuring instruments, and formal test methods which define the instrument's use, are the means by which these relations of numbers are obtained. All measuring instruments are subject to varying degrees of instrument error and measurement uncertainty.
Scientists, engineers and other humans use a vast range of instruments to perform their measurements. These instruments may range from simple objects such as rulers and stopwatches to electron microscopes and particle accelerators. Virtual instrumentation is widely used in the development of modern measuring instruments.
Main articles: Metrology and History of measurementFurther information: List of measuring devices and List of sensorsTime-points in the past can be measured with respect to the present of an observer. Time-points in the future can be fixed. But there seems to exist no device that can set time to a predetermined value (time machine), like it is possible with other physical quantities (for example: distance or volume). The time-point called present seems to move in one direction only, the future. Entropy production and cause-and-effect observations of events correlate to this observation.
For more information on time, especially standards, also consult the time portal.
- Atomic clock
- Calendar (by counting days)
- Chronometer, Chronograph
- Clock
- Egg timer
- Wall clock
- Hourglass
- Pendulum clock
- Radio clock
- Radiometric dating
- Stopwatch
- Sundial
- Transit telescope
- Water clock
Timeline of time measurement technology
For the ranges of time-values see: Orders of magnitude (time)
Energy
Main article: Energy Changing energy carriers, linear momentum to angular momentum. No measurement primarily intended.
Changing energy carriers, linear momentum to angular momentum. No measurement primarily intended.
Example: In a plant that furnishes pumped-storage hydroelectricity, mechanical work and electrical work is done by machines like electric pumps and electrical generators. The pumped water stores mechanical work. The amount of energy put into the system equals the amount of energy which comes out of the system, less that amount of energy used to overcome friction.
Such examples suggested the derivation of some unifying concepts: Instead of discerning (transferred) forms of work or stored work, there has been introduced one single physical quantity called energy. Energy is assumed to have substance-like qualities; energy can be apportioned and transferred. Energy cannot be created from nothing, or to be annihilated to nothing, thus energy becomes a conserved quantity, when properly balanced.
Describing the transfer of energy two dictions, two ways of wording are used:
(energy carriers exchanging energy) Physical interactions occur by carriers (linear momentum, electric charge, entropy) exchanging energy. For example, a generator transfers energy from angular momentum to electric charge.[1]
(energy forms transforming energy) Energy forms are transformed; for example mechanical energy into electrical energy by a generator.[2]
Often the energy value results from multiplying two related quantities: (a generalized) potential (relative velocity, voltage, temperature difference) times some substance-like quantity (linear momentum, electrical charge, entropy). — Thus energy has to be measured by first choosing a carrier/form. The measurement usually happens indirectly, by obtaining two values (potential and substance-like quantity) and by multiplying their values.
- (see any measurement device for energy below)
For the ranges of energy-values see: Orders of magnitude (energy)
Power (flux of energy)
Main article: Power (physics)A physical system that exchanges energy may be described by the amount of energy exchanged per time-interval, also called power or flux of energy.
- (see any measurement device for power below)
For the ranges of power-values see: Orders of magnitude (power).
Action
Main article: Action (physics)Action describes energy summed up over the time a process lasts (time integral over energy). Its dimension is the same as that of an angular momentum.
- A phototube provides a voltage measurement which permits the calculation of the quantized action (Planck constant) of light. Also see photoelectric effect.
Mechanics
This includes basic quantities found in Classical- and continuum mechanics; but strives to exclude temperature-related questions or quantities.
Length (distance)
See also: Distance measuring equipmentFor the ranges of length-values see: Orders of magnitude (length)
- Altimeter, height
- Architect's scale
- Caliper
- Electronic distance meter
- Engineer's scale
- Frequency comb
- Gauge blocks
- GPS, indirect by runtime measurement of electromagnetic waves in the GHz-range
- Interferometer
- Laser rangefinder, indirect by runtime measurement of coherent electromagnetic waves around the visible light region (lidar)
- Metric scale
- Micrometer
- Odometer
- Opisometer
- Feeler gauge, used in metal working to measure size of gaps
- Radar antenna, indirect by runtime measurement of electromagnetic waves around the microwave region (radar)
- Ruler
- Surveyor's wheel
- Tachymeter
- Tape measure
- Taximeter, measure usually includes a time component as well
- Travelling microscope
- Ultrasound distance measure, indirect by runtime measurement of sound waves (sonar, Echo sounding)
- Urethra gauge, cylindrical circumferencial measurement device.
Area
For the ranges of area-values see: Orders of magnitude (area)
Volume
 A measuring cup, a common instrument used to measure volume.
A measuring cup, a common instrument used to measure volume.
- buoyant weight (solids)
- overflow trough (solids)
- Measuring cup (grained solids, liquids)
- Flow measurement devices (liquids)
- Graduated cylinder (liquids)
- Pipette (liquids)
- Eudiometer, pneumatic trough (gases)
(if the mass density of a solid is known, weighing allows to calculate the volume)
For the ranges of volume-values see: Orders of magnitude (volume)
Mass- or volume flow measurement
Speed (flux of length)
- Airspeed indicator
- Radar gun, a Doppler radar device, using the Doppler effect for indirect measurement of velocity.
- Speedometer
- Tachometer (speed of rotation)
- Tachymeter
- Variometer
For the ranges of speed-values see: Orders of magnitude (speed)
Acceleration
Mass
A pair of scales: An instrument for measuring mass in a force field by balancing forces.
- Balance
- Automatic checkweighing machines
- Katharometer
- Weighing scales
- Inertial balance
- Mass spectrometers measure the mass-to-charge ratio, not the mass
For the ranges of mass-values see: Orders of magnitude (mass)
Linear momentum
Force (flux of linear momentum)
- Force gauge
- Spring scale
- Strain gauge
- Torsion balance
- Tribometer
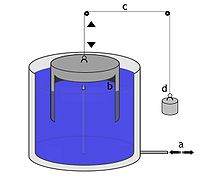
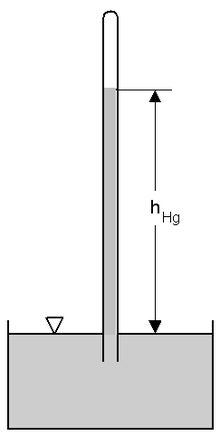 Measuring absolute pressure in an accelerated reference frame: The principle of a mercury (Hg) barometer in the gravitational field of the earth.
Measuring absolute pressure in an accelerated reference frame: The principle of a mercury (Hg) barometer in the gravitational field of the earth.
Pressure (flux density of linear momentum)
- Anemometer (used to determine wind speed)
- Barometer used to measure the atmospheric pressure.
- Manometer see pressure measurement
- Pitot tube (used to determine speed)
- Tire-pressure gauge in industry and mobility
For the ranges of pressure-values see: Orders of magnitude (pressure)
Timeline of temperature and pressure measurement technology
Angle
- Circumferentor
- Cross staff
- Goniometer
- Graphometer
- Protractor
- Quadrant
- Reflecting instruments
- Theodolite
Angular velocity or rotations per time unit
For the value-ranges of angular velocity see: Orders of magnitude (angular velocity)
For the ranges of frequency see: Orders of magnitude (frequency)
Torque
Orientation in three-dimensional space
See also the section about navigation below.
Level
Direction
Energy carried by mechanical quantities, mechanical work
- Ballistic pendulum, indirectly by calculation and or gauging
Electricity, electronics and electrical engineering
Considerations related to electric charge dominate electricity and electronics. Electrical charges interact via a field. That field is called electric if the charge doesn't move. If the charge moves, thus realizing an electric current, especially in an electrically neutral conductor, that field is called magnetic. Electricity can be given a quality — a potential. And electricity has a substance-like property, the electric charge. Energy (or power) in elementary electrodynamics is calculated by multiplying the potential by the amount of charge (or current) found at that potential: potential times charge (or current). (See Classical electromagnetism and its Covariant formulation of classical electromagnetism)
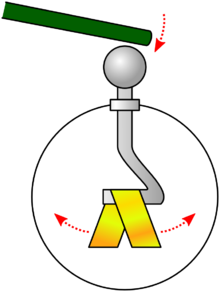 An instrument for detecting net charges, the electroscope.
An instrument for detecting net charges, the electroscope.
Electric charge
- Electrometer is often used to reconfirm the phenomenon of contact electricity leading to triboelectric sequences.
- Torsion balance used by Coulomb to establish a relation between charges and force, see above.
For the ranges of charge values see: Orders of magnitude (charge) df
Electric current (current of charge)
- Ammeter
- Clamp meter
- Galvanometer
Voltage (electric potential difference)
- Oscilloscope allows to quantify time depended voltages
- Voltmeter
Electric resistance, electrical conductance (and electrical conductivity)
- Ohmmeter
- Time-domain reflectometer characterizes and locates faults in metallic cables by runtime measurements of electric signals.
- Wheatstone bridge
Electric capacitance
Electric inductance
- Inductance meter
Energy carried by electricity or electric energy
- Electric energy meter
- Electricity meter
Power carried by electricity (current of energy)
- These are instruments used for measuring electrical properties. Also see meter (electronics).
Electric field (negative gradient of electric potential, voltage per length)
Magnetic field
See also the relevant section in the article about the magnetic field.
For the ranges of magnetic field see: Orders of magnitude (magnetic field)
Combination instruments
- Multimeter, combines the functions of ammeter, voltmeter and ohmmeter as a minimum.
- LCR meter, combines the functions of ohmeter, capacitance meter and inductance meter. Also called component bridge due to the bridge circuit method of measurement.
Thermodynamics
Temperature-related considerations dominate thermodynamics. There are two distinct thermal properties: A thermal potential — the temperature. For example: A glowing coal has a different thermal quality than a non-glowing one.
And a substance-like property, — the entropy; for example: One glowing coal won't heat a pot of water, but a hundred will.
Energy in thermodynamics is calculated by multipying the thermal potential by the amount of entropy found at that potential: temperature times entropy.
Entropy can be created by friction but not annihilated.
Amount of substance (or mole number)
- A physical quantity introduced in chemistry; usually determined indirectly. If mass and substance type of the sample are known, then atomic- or molecular masses (taken from a periodic table, masses measured by mass spectrometry) give direct access to the value of the amount of substance. See also the article about molar masses. If specific molar values are given, then the amount of substance of a given sample may be determined by measuring volume, mass or concentration. See also the subsection below about the measurement of the boiling point.
- Gas collecting tube gases
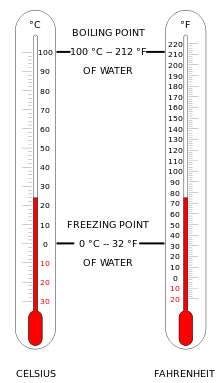
Temperature
- Electromagnetic spectroscopy
- Galileo thermometer
- Gas thermometer principle: relation between temperature and volume or pressure of a gas (Gas laws).
- constant pressure gas thermometer
- constant volume gas thermometer
- Liquid crystal thermometer
- liquid thermometer principle: relation between temperature and volume of a liquid (Coefficient of thermal expansion).
- Pyranometer principle: solar radiation flux density relates to surface temperature (Stefan–Boltzmann law)
- Pyrometers principle: temperature dependence of spectral intensity of light (Planck's law), i.e. the color of the light relates to the temperature of its source, range: from about −50 °C to +4000 °C, note: measurement of thermal radiation (instead of thermal conduction, or thermal convection) means: no physical contact becomes necessary in temperature measurement (pyrometry). Also note: thermal space resolution (images) found in Thermography.
- Resistance thermometer principle: relation between temperature and electrical resistance of metals (platinum) (Electrical resistance), range: 10 to 1,000 kelvins, application in physics and industry
- solid thermometer principle: relation between temperature and length of a solid (Coefficient of thermal expansion).
- Thermistors principle: relation between temperature and electrical resistance of ceramics or polymers, range: from about 0.01 to 2,000 kelvins (−273.14 to 1,700 °C)
- Thermocouples principle: relation between temperature and voltage of metal junctions (Seebeck effect), range: from about −200 °C to +1350 °C
- Thermometer
- Thermopile is a set of connected thermocouples
- Triple Point cell used for calibrating thermometers.
Imaging technology
- Thermographic camera uses a microbolometer for detection of heat-radiation.
See also Temperature measurement and Category:Thermometers. More technically related may be seen thermal analysis methods in materials science.
For the ranges of temperature-values see: Orders of magnitude (temperature)
Energy carried by entropy or thermal energy
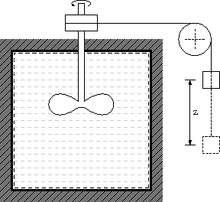 An active calorimeter lacking a temperature measurement device.
An active calorimeter lacking a temperature measurement device.
This includes thermal capacitance or temperature coefficient of energy, reaction energy, heat flow ... Calorimeters are called passive if gauged to measure emerging energy carried by entropy, for example from chemical reactions. Calorimeters are called active or heated if they heat the sample, or reformulated: if they are gauged to fill the sample with a defined amount of entropy.
- Actinometer measures the heating power of radiation.
- constant-temperature calorimeter, phase change calorimeter for example an ice calorimeter or any other calorimeter observing a phase change or using a gauged phase change for heat measurement.
- constant-volume calorimeter, also called bomb calorimeter
- constant-pressure calorimeter, enthalpy-meter or coffee cup calorimeter
- Differential Scanning Calorimeter
- Reaction calorimeter
- see also Calorimeter or Calorimetry
Entropy
Entropy is accessible indirectly by measurement of energy and temperature.
Entropy transfer
Phase change calorimeter's energy value divided by absolute temperature give the entropy exchanged. Phase changes produce no entropy and therefore offer themselves as an entropy measurement concept. Thus entropy values occur indirectly by processing energy measurements at defined temperatures, without producing entropy.
- constant-temperature calorimeter, phase change calorimeter
- Heat flux sensor uses thermopiles which are connected thermocouples to determine current density or flux of entropy.
Entropy content
The given sample is cooled down to (almost) absolute zero (for example by submerging the sample in liquid helium). At absolute zero temperature any sample is assumed to contain no entropy (see Third law of thermodynamics for further information). Then the following two active calorimeter types can be used to fill the sample with entropy until the desired temperature has been reached: (see also Thermodynamic databases for pure substances)
- constant-pressure calorimeter, enthalpy-meter, active
- constant-temperature calorimeter, phase change calorimeter, active
Entropy production
Processes transferring energy from a non-thermal carrier to heat as a carrier do produce entropy (Example: mechanical/electrical friction, established by Count Rumford). Either the produced entropy or heat are measured (calorimetry) or the transferred energy of the non-thermal carrier may be measured.
- calorimeter
- (any device for measuring the work which will or would eventually be converted to heat and the ambient temperature)
Entropy lowering its temperature—without losing energy—produces entropy (Example: Heat conduction in an isolated rod; "thermal friction").
- calorimeter
temperature coefficient of energy or "heat capacity"
Concerning a given sample, a proportionality factor relating temperature change and energy carried by heat. If the sample is a gas, then this coefficient depends significantly on being measured at constant volume or at constant pressure. (The terminiology preference in the heading indicates that the classical use of heat bars it from having substance-like properties.)
- constant-volume calorimeter, bomb calorimeter
- constant-pressure calorimeter, enthalpy-meter
specific temperature coefficient of energy or "specific heat"
The temperature coefficient of energy divided by a substance-like quantity (amount of substance, mass, volume) describing the sample. Usually calculated from measurements by a division or could be measured directly using a unit amount of that sample.
For the ranges of specific heat capacities see: Orders of magnitude (specific heat capacity)
Coefficient of thermal expansion
Melting temperature (of a solid)
- Thiele tube
- Kofler bench
- Differential Scanning Calorimeter gives melting point and enthalpy of fusion.
Boiling temperature (of a liquid)
- Ebullioscope a device for measuring the boiling point of a liquid. This device is also part of a method that uses the effect of boiling point elevation for calculating the molecular mass of a solvent.
See also thermal analysis, Heat.
More on continuum mechanics
This includes mostly instruments which measure macroscopic properties of matter: In the fields of solid state physics; in condensed matter physics which considers solids, liquids and in-betweens exhibiting for example viscoelastic behavior. Furthermore fluid mechanics, where liquids, gases, plasmas and in-betweens like supercritical fluids are studied.
Density
This refers to particle density of fluids and compact(ed) solids like crystals, in contrast to bulk density of grainy or porous solids.
- Aerometer liquids
- Dasymeter gases
- Gas collecting tube gases
- Hydrometer liquids
- Pycnometer liquids
- resonant frequency and Damping Analyser (RFDA) solids
For the ranges of density-values see: Orders of magnitude (density)
Hardness of a solid
- Durometer
Shape and surface of a solid
- Holographic interferometer
- Laser produced speckle pattern analysed.
- resonant frequency and Damping Analyser (RFDA)
- Tribometer
Deformation of condensed matter
- Strain gauge all below
Elasticity of a solid (Elastic moduli)
- resonant frequency and Damping Analyser (RFDA), using the impulse excitation technique: A small mechanical impulse causes the sample to vibrate. The vibration depends on elastic properties, density, geometry and inner structures (lattice or fissures).
Plasticity of a solid
Tensile strength, ductility or malleability of a solid
Granularity of a solid or of a suspension
Viscosity of a fluid
Optical activity
Surface tension of liquids
- Tensiometer
Imaging technology
- Tomograph, device and method for non-destructive analysis of multiple measurements done on a geometric object, for producing 2- or 3-dimensional images, representing the inner structure of that geometric object.
- Wind tunnel
This section and the following sections include instruments from the wide field of Category:Materials science, materials science.
More on electric properties of condensed matter, gas
Permittivity, relative static permittivity, (dielectric constant) or electric susceptibility
Such measurements also allow to access values of molecular dipoles.
Magnetic susceptibility or magnetization
For other methods see the section in the article about magnetic susceptibility.
See also the Category:Electric and magnetic fields in matter
Substance potential or chemical potential or molar Gibbs energy
Phase conversions like changes of aggregate state, chemical reactions or nuclear reactions transmuting substances, from reactants to products, or diffusion through membranes have an overall energy balance. Especially at constant pressure and constant temperature molar energy balances define the notion of a substance potential or chemical potential or molar Gibbs energy, which gives the energetic information about whether the process is possible or not - in a closed system.
Energy balances that include entropy consist of two parts: A balance that accounts for the changed entropy content of the substances. And another one that accounts for the energy freed or taken by that reaction itself, the Gibbs energy change. The sum of reaction energy and energy associated to the change of entropy content is also called enthalpy. Often the whole enthalpy is carried by entropy and thus measurable calorimetrically.
For standard conditions in chemical reactions either molar entropy content and molar Gibbs energy with respect to some chosen zero point are tabulated. Or molar entropy content and molar enthalpy with respect to some chosen zero are tabulated. (See Standard enthalpy change of formation and Standard molar entropy)
The substance potential of a redox reaction is usually determined electrochemically current-free using reversible cells.
Other values may be determined indirectly by calorimetry. Also by analyzing phase-diagrams.
See also the article on electrochemistry.
Sub-microstructural properties of condensed matter, gas
- Infrared spectroscopy
- Neutron detector
- Radio frequency spectrometers for Nuclear magnetic resonance and for Electron paramagnetic resonance
- Raman spectroscopy
Crystal structure
- An X-ray tube, a sample scattering the X-rays and a photographic plate to detect them. This constellation forms the scattering instrument used by X-ray crystallography for investigating crystal structures of samples. Amorphous solids lack a distinct pattern and are identifyable thereby.
Imaging technology, Microscope
- Electron microscope
- Transmission electron microscope
- Optical microscope uses reflectiveness or refractiveness of light to produce an image.
- Scanning acoustic microscope
- Scanning probe microscope
- Focus variation
- X-ray microscope
See also the article on spectroscopy and the list of materials analysis methods.
Rays ("waves" and "particles")
Sound, compression waves in matter
Microphones in general, sometimes their sensitivity is increased by the reflection- and concentration principle realized in acoustic mirrors.
Sound pressure
- microphone or hydrophone properly gauged
- Shock tube
- Sound level meter
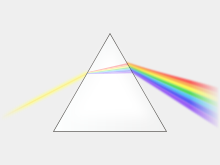 A device for unmixing sun-light: the prism.
A device for unmixing sun-light: the prism.
Light and radiation without a rest mass, non-ionizing
- Antenna (radio)
- bolometer measuring the energy of incident electromagnetic radiation.
- camera
- EMF meter
- Interferometer used in the wide field of Interferometry
- Optical power meter
- Microwave power meter
- Photographic plate
- Photomultiplier
- Phototube
- Radio telescope
- Spectrometer
- T-ray detectors
(for lux meter see the section about human senses and human body)
See also Category:Optical devices
Photon polarization
Pressure (current density of linear momentum)
radiant flux
The measure of the total power of light emitted.
- Integrating sphere for measuring the total radiant flux of a light source
Radiation with a rest mass, particle radiation
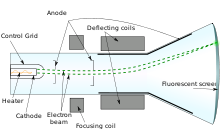
Cathode ray
- Crookes tube
- Cathode ray tube, a phosphor coated anode
Atom polarization and electron polarization
- Stern-Gerlach experiment
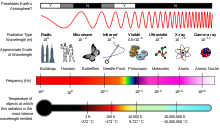
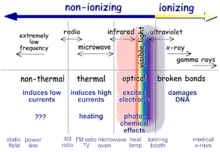 Another visualization of the electromagnetic spectrum.
Another visualization of the electromagnetic spectrum.
Ionizing radiation
Ionizing radiation includes rays of "particles" as well as rays of "waves". Especially X-rays and Gamma rays transfer enough energy in non-thermal, (single) collision processes to separate electron(s) from an atom.
particle flux
- Bubble chamber
- Cloud chamber
- Dosimeter, a technical device realizes different working principles.
- Geiger counter
- Microchannel plate detector
- Photographic plate
- Photostimulable phosphors
- Scintillation counter, Lucas cell
- Semiconductor detector
Identification and content
This could include chemical substances, rays of any kind, elementary particles, quasiparticles. Many measurement devices outside this section may be used or at least become part of an identification process. For identification and content concerning chemical substances see also analytical chemistry especially its List of chemical analysis methods and the List of materials analysis methods.
Substance content in mixtures, substance identification
- Carbon dioxide sensor
- chromatographic device, gas chromatograph separates mixtures of substances. Different velocites of the substance types accomplish the separation.
- Colorimeter (measures absorbance, and thus concentration)
- gas detector
- Gas detector in combination with mass spectrometer,
- mass spectrometer identifies the chemical composition of a sample on the basis of the mass-to-charge ratio of charged particles.
- Nephelometer or turbidimeter
- oxygen sensor (= lambda sond)
- Refractometer, indirectly by determining the refractive index of a substance.
- Smoke detector
- Ultracentrifuge, separates mixtures of substances. In a force field of a centrifuge, substances of different densities separate.
pH: Concentration of protons in a solution
Humidity
- Hygrometer measures the density of water in air
- Lysimeter measures the balance of water in soil
Human senses and human body
Sight
Luminuos flux, photometry
A measure of the perceived power of light, luminous flux is adjusted to reflect the varying sensitivity of the human eye to different wavelengths of light.
- Integrating sphere for measuring the total luminuos flux of a light source
illuminance, photometry
- Densitometer
- Light meter
- Lux meter
- Photometer
Hearing
Loudness in phon
- Headphone, loudspeaker, sound pressure gauge, for measuring an equal-loudness contour of a human ear.
- Sound level meter calibrated to an equal-loudness contour of the human auditory system behind the human ear.
Smell
- Olfactometer, see also the article about olfaction.
Temperature (sense and body)
Body temperature or Core temperature
- Medical thermometer, see also infrared thermometer
circulatory system (mainly heart and blood vessels for distributing substances fast)
Blood-related parameters are listed in a blood test.
- Electrocardiograph records the electrical activity of the heart
- Glucose meter for obtaining the status of blood sugar.
- Sphygmomanometer, a blood pressure meter used to determine blood pressure in medicine. See also Category:Blood tests
Respiratory system (lung and airways controlling the breathing process)
concentration or partial pressure of carbon dioxide in the respiratory gases
nervous system (nerves transmitting and processing information electrically)
- Electroencephalograph records the electrical activity of the brain
musculoskeletal system (muscles and bones for movement)
power, work of muscles
metabolic system
Medical imaging
- Computed tomography
- Magnetic resonance imaging
- Medical ultrasonography
- Radiology
- Tomograph, device and method for non-destructive analysis of multiple measurements done on a geometric object, for producing 2- or 3-dimensional images, representing the inner structure of that geometric object.
See also: Category:Physiological instruments and Category:Medical testing equipment.
Meteorology
See also Category:Meteorological instrumentation and equipment.
See also Category:Navigational equipment and Category:Navigation. See also Category:Surveying instruments.
Astronomy
See also Category:Astronomical instruments and Category:Astronomical observatories.
Military
Some instruments, such as telescopes and sea navigation instruments, have had military applications for many centuries. However, the role of instruments in military affairs rose exponentially with the development of technology via applied science, which began in the mid-19th century and has continued through the present day. Military instruments as a class draw on most of the categories of instrument described throughout this article, such as navigation, astronomy, optics and imaging, and the kinetics of moving objects. Common abstract themes that unite military instruments are seeing into the distance, seeing in the dark, knowing an object's geographic location, and knowing and controlling a moving object's path and destination.
Special features of these instruments may include ease of use, speed, reliability and accuracy; nevertheless additionally one might hope seeing them as instruments whose existence, not use, ultimately helps in establishing a humane and humanistic peace between individual humans as well as groups of them.
Uncategorized, specialized, or generalized application
- Checkweigher measures precise weight of items in a conveyor line, rejecting under or overweight objects.
- Densitometer measures light transmission through processed photographic film or transparent material or light reflection from a reflective material.
- Force platform measures ground reaction force.
- Gauge (engineering) A highly precise measurement instrument, also usable to calibrate other instruments of the same kind. Often found in conjunction with defining or applying technical standards.
- Gradiometer any device that measures spatial variations of a physical quantity. For example as done in gravity gradiometry.
- Parking meter measures time a vehicle is parked at a particular spot, usually with a fee.
- Postage meter measures postage used from a prepaid account.
- S meter measures the signal strength processed by a communications receiver.
- Sensor, hypernym for devices that measure with little interaction, typically used in technical applications.
- Spectroscope is an important tool used by physicists.
- SWR meter check the quality of the match between the antenna and the transmission line.
- Time-domain reflectometer locates faults in metallic cables.
- Universal measuring machine measures geometric locations for inspecting tolerances.
Fictional devices
- Tricorder, a multipurpose scanning device, originating from the science-fictional Star Trek series.
- Sonic Screwdriver, a multifunctional device used occasionally for scanning, originating from the science-fictional Doctor Who series.
See also
- Category:Instrument-making corporations
- Detectors
- History of weights and measures
- Instrumentation
- List of measuring devices for a more comprehensive, alphabetical list of devices and the corresponding list of physical quantities.
- Metrology
- Timeline of temperature and pressure measurement technology
Notes
Note that the alternate spelling "-metre" is never used when referring to a measuring device.
References
Categories:- Measuring instruments
- Metrology
Wikimedia Foundation. 2010.