- Microstructure
-
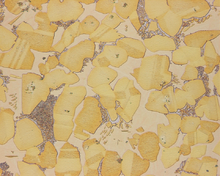
Microstructure is defined as the structure of a prepared surface or thin foil of material as revealed by a microscope above 25× magnification.[1] The microstructure of a material (which can be broadly classified into metallic, polymeric, ceramic and composite) can strongly influence physical properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low temperature behavior, wear resistance, and so on, which in turn govern the application of these materials in industrial practice.
Contents
Methods
The concept of microstructure is perhaps more accessible to the casual observer through macrostructural features in commonplace objects. If one ever comes across a piece of galvanized steel, such as the casing of a lamp post or road divider, one observes that the surface is not uniformly colored, but is covered with a patchwork of interlocking polygons of different shades of grey or silver. Each polygon (the most frequently occurring would be hexagons) is a single crystal of zinc adhering to the surface of the steel beneath. Zinc and lead are two common metals which form large crystals visible to the naked eye. The metallic atoms in each crystal are well-organized into one of seven crystal lattice systems possible for metals (cubic, tetrahedral, hexagonal, monoclinic, triclinic, rhombohedral, orthorhombic); these systems dictate that the atoms are all lined up like points in a 3-D matrix. However, the direction of alignment of the matrices differ from crystal to adjacent crystal, leading to variance in the reflectivity of each presented face of the interlocked crystals on the galvanized surface. Symmetrical crystals are generally unstressed, unworked. They grow in all directions equally and were not subjected to deforming stresses either during or after. For large crystals, the ratio of crystal bulk to inter-crystal boundary (more properly, intergranullar boundary) is high. This indicates high ductility but correspondingly, lower strength (see Hall-Petch Strengthening), but a true study would take into quantitative account the relative strengths of the crystal and that of inter-crystal bonding.
Microscopy
Optical
When a polished flat sample reveals traces of its microstructure, it is normal to capture the image using macrophotography. More sophisticated microstructure examination involves higher powered instruments: optical microscopy, electron microscopy, X-ray diffraction and so on, some involving preparation of the material sample (cutting, microtomy, polishing, etching, vapor-deposition etc.). The methods are known collectively as metallography as applied to metals and alloys, and can be used in modified form for any other material, such as ceramics, glasses, composites, and polymers.
Two kinds of optical microscope are generally used to examine flat, polished and etched specimens: a reflection microscope and an inverted microscope. Recording the image is achieved using a digital camera working through the eyepiece.
X-Ray microtomographic
See also: Industrial CT ScanningNondestructive testing of microstructure for biological materials is a challenge and computer microtomography is the current solution. In fact, CMT can be used for the evaluation of microstructure of many other materials also. CMT can be very expensive though, and for research purposes, it is a necessity to generate a three-dimensional microstructure from two-dimensional cross-sectional images of the material. This is an area of active research and pursued by many scientists.
Electron microscopy
See also: Electron microscopyFor high-resolution information on metallurgical microstructures, electron microscopic methods can be employed. This can allow for direct observation of atomic-scale features such as very fine precipitation reactions, dislocations or grain-boundary interfaces. Such methods may be critical in determining parameters such as solid state diffusivities.
See also
References
- ^ Adapted from ASM Metals Handbook, Ninth Edition, v. 9, "Metallography and Microstructures", American Society for Metals, Metals Park, OH, 1985, p. 12.
Categories:
Wikimedia Foundation. 2010.