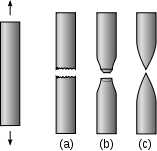- Ductility
-
In materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized by the material's ability to form a thin sheet by hammering or rolling. Both of these mechanical properties are aspects of plasticity, the extent to which a solid material can be plastically deformed without fracture. Also, these material properties are dependent on temperature and pressure (investigated by Percy Williams Bridgman as part of his Nobel Prize winning work on high pressures).
Ductility and malleability are not always coextensive – for instance, while gold is both ductile and malleable, lead is only malleable.[1] The word ductility is sometimes used to embrace both types of plasticity.[2]
Contents
Materials science
Ductility is especially important in metalworking, as materials that crack or break under stress cannot be manipulated using metal forming processes, such as hammering, rolling, and drawing. Malleable materials can be formed using stamping or pressing, whereas brittle metals and plastics must be molded.
High degrees of ductility occur due to metallic bonds, which are found predominantly in metals and leads to the common perception that metals are ductile in general. In metallic bonds valence shell electrons are delocalized and shared between many atoms. The delocalized electrons allow metal atoms to slide past one another without being subjected to strong repulsive forces that would cause other materials to shatter.
Ductility can be quantified by the fracture strain εf, which is the engineering strain at which a test specimen fractures during a uniaxial tensile test. Another commonly used measure is the reduction of area at fracture q.[3]
The following list ranks metals from the greatest ductility to least: gold, silver, platinum, iron, nickel, copper, aluminium, zinc, tin, and lead.[1] The malleability of the same metals are then ranked from greatest to least: gold, silver, lead, copper, aluminium, tin, platinum, zinc, iron, and nickel.[1] The ductility of steel varies depending on the alloying constituents. Increasing levels of carbon decreases ductility. Many plastics and amorphous solids, such as Play-Doh, are also malleable.
Ductile-brittle transition temperature
The ductile-brittle transition temperature (DBTT), nil ductility temperature (NDT), or nil ductility transition temperature of a metal represents the point at which the fracture energy passes below a pre-determined point (for steels typically 40 J[4] for a standard Charpy impact test). DBTT is important since, once a material is cooled below the DBTT, it has a much greater tendency to shatter on impact instead of bending or deforming. For example, zamak 3 exhibits good ductility at room temperature but shatters at sub-zero temperatures when impacted. DBTT is a very important consideration in materials selection when the material in question is subject to mechanical stresses. A similar phenomenon, the glass transition temperature, occurs with glasses and polymers, although the mechanism is different in these amorphous materials.
In some materials this transition is sharper than others. For example, the transition is generally sharper in materials with a body-centered cubic (BCC) lattice than those with a face-centered cubic (FCC) lattice. DBTT can also be influenced by external factors such as neutron radiation, which leads to an increase in internal lattice defects and a corresponding decrease in ductility and increase in DBTT.
The most accurate method of measuring the BDT or DBT temperature of a material is by fracture testing. Typically, four point bend testing at a range of temperatures is performed on pre-cracked bars of polished material. For experiments conducted at higher temperatures, dislocation activity increases. At a certain temperature, dislocations shield the crack tip to such an extent the applied deformation rate is not sufficient for the stress intensity at the crack-tip to reach the critical value for fracture (KiC). The temperature at which this occurs is the ductile-brittle transition temperature. If experiments are performed at a higher strain rate, more dislocation shielding is required to prevent brittle fracture and the transition temperature is raised.
See also
- Deformation
- Work hardening, which reduces ductility
References
- ^ a b c Rich, Jack C. (1988). The Materials and Methods of Sculpture. Courier Dover Publications. p. 129. ISBN 0486257428. http://books.google.com/?id=hW13qhOFa7gC.
- ^ "Ductile". TheFreeDictionary.com. Farlex. http://www.thefreedictionary.com/ductile. Retrieved January 30, 2011. Includes definitions from American Heritage Dictionary of the English Language, Collins English Dictionary: Complete and Unabridged, American Heritage Science Dictionary, and WordNet 3.0.
- ^ G. Dieter, Mechanical Metallurgy, McGraw-Hill, 1986, ISBN 978-0070168930
- ^ John, Vernon. Introduction to Engineering Materials, 3rd ed.(?) New York: Industrial Press, 1992. ISBN 0831130431.
External links
Categories:
Wikimedia Foundation. 2010.