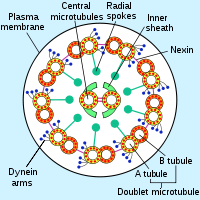
- Dynein
-
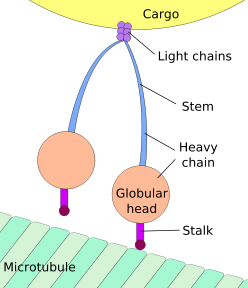 Cytoplasmic dynein has two heavy chains with globular "heads" that "walk" along the microtubule, to which they are bound by the "stalks". Dynactin (not shown) may help attach the light chains to the cargo. Interactions between the "stalks" and the microtubule must repeatedly form and break (see main text for details)
Cytoplasmic dynein has two heavy chains with globular "heads" that "walk" along the microtubule, to which they are bound by the "stalks". Dynactin (not shown) may help attach the light chains to the cargo. Interactions between the "stalks" and the microtubule must repeatedly form and break (see main text for details)
Dynein is a motor protein (also called molecular motor or motor molecule) in cells which converts the chemical energy contained in ATP into the mechanical energy of movement. Dynein transports various cellular cargo by "walking" along cytoskeletal microtubules towards the minus-end of the microtubule, which is usually oriented towards the cell center. Thus, they are called "minus-end directed motors," while kinesins, motor proteins that move toward the microtubules' plus end, are called plus-end directed motors.
Contents
Classification
Dynein heavy chain, N-terminal region 1 Identifiers Symbol DHC_N1 Pfam PF08385 InterPro IPR013594 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Dynein heavy chain, N-terminal region 2 Identifiers Symbol DHC_N2 Pfam PF08393 InterPro IPR013602 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Dynein heavy chain and region D6 of dynein motor Identifiers Symbol Dynein_heavy Pfam PF03028 InterPro IPR004273 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Dynein light intermediate chain (DLIC) Identifiers Symbol DLIC Pfam PF05783 Pfam clan CL0023 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Dynein light chain type 1 
structure of the human pin/lc8 dimer with a bound peptide Identifiers Symbol Dynein_light Pfam PF01221 InterPro IPR001372 PROSITE PDOC00953 SCOP 1bkq Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Dyneins can be divided into two groups: cytoplasmic dyneins and axonemal dyneins, which are also called ciliary or flagellar dyneins.
- axonemal
- cytoplasmic
Function
Axonemal dynein causes sliding of microtubules in the axonemes of cilia and flagella and is found only in cells that have those structures. Cytoplasmic dynein, found in all animal cells and possibly plant cells as well, performs functions necessary for cell survival such as organelle transport and centrosome assembly.[1]
Cytoplasmic dynein moves processively along the microtubule; that is, one or the other of its stalks is always attached to the microtubule so that the dynein can "walk" a considerable distance along a microtubule without detaching.
Cytoplasmic dynein probably helps to position the Golgi complex and other organelles in the cell.[1] It also helps transport cargo needed for cell function such as vesicles made by the endoplasmic reticulum, endosomes, and lysosomes (Karp, 2005). Dynein is also probably involved in the movement of chromosomes and positioning the mitotic spindles for cell division.[1] Dynein carries organelles and microtubule fragments along the axons of neurons in a process called axoplasmic transport [1]).
Structure
Each molecule of the dynein motor is a complex protein assembly composed of many smaller polypeptide subunits. Cytoplasmic and axonemal dynein contain some of the same components, but they also contain some unique subunits.
Cytoplasmic dynein
Cytoplasmic dynein, which has a molecular mass of about 1.5 Megadaltons (MDa), contains approximately twelve polypeptide subunits: two identical "heavy chains," 520 kDa in mass, which contain the ATPase activity and are thus responsible for generating movement along the microtubule; two 74 kDa intermediate chains which are believed to anchor the dynein to its cargo; four 53-59 kDa intermediate chains and several light chains which are less understood.
The force-generating ATPase activity of each dynein heavy chain is located in its large doughnut-shaped "head", which is related to other AAA proteins, while two projections from the head connect it to other cytoplasmic structures. One projection, the coiled-coil stalk, binds to and "walks" along the surface of the microtubule via a repeated cycle of detachment and reattachment. The other projection, the extended tail (also called "stem"), binds to the intermediate and light chain subunits which attach the dynein to its cargo. The alternating activity of the paired heavy chains in the complete cytoplasmic dynein motor enables a single dynein molecule to transport its cargo by "walking" a considerable distance along a microtubule without becoming completely detached.
In eukaryotes, cytoplasmic dynein must be activated by binding of dynactin, another multisubunit protein that is essential for mitosis. Dynactin may regulate the activity of dynein, and possibly facilitates the attachment of dynein to its cargo.
Axonemal dynein
Axonemal dynein come in multiple forms that contain either one, two or three non-identical heavy chains (depending upon the organism and location in the cilium). Each heavy chain has a globular motor domain with a doughnut-shaped structure believed to resemble that of other AAA proteins, a coiled coil "stalk" that binds to the microtubule, and an extended tail (or "stem") that attaches to a neighboring microtubule of the same axoneme. Each dynein molecule thus forms a cross-bridge between two adjacent microtubules of the ciliary axoneme. During the "power stroke", which causes movement, the AAA ATPase motor domain undergoes a conformational change that causes the microtubule-binding stalk to pivot relative to the cargo-binding tail with the result that one microtubule slides relative to the other (Karp, 2005). This sliding produces the bending movement needed for cilia to beat and propel the cell or other particles. Groups of dynein molecules responsible for movement in opposite directions are probably activated and inactivated in a coordinated fashion so that the cilia or flagella can move back and forth. The radial spoke has been proposed as the (or one of the) structures that synchronizes this movement.
History
The protein responsible for movement of cilia and flagella was first discovered and named dynein in 1963 (Karp, 2005). 20 years later, cytoplasmic dynein, which had been suspected to exist since the discovery of flagellar dynein, was isolated and identified (Karp, 2005).
See also
- Molecular motors
References
- ^ a b c d Gerald Karp, Kurt Beginnen, Sebastian Vogel, Susanne Kuhlmann-Krieg (2005) (in fr). Molekulare Zellbiologie. Springer. ISBN 9783540238577. http://books.google.com/?id=ELrrMbschQgC.
- Karp G. (2005). Cell and Molecular Biology: Concepts and Experiments (4th ed.). Hoboken, NJ: John Wiley and Sons. pp. 346–358. ISBN 0471192791.
- Schroer TA (2004). "Dynactin". Annu. Rev. Cell Dev. Biol. 20: 759–79. doi:10.1146/annurev.cellbio.20.012103.094623. PMID 15473859. http://arjournals.annualreviews.org/doi/full/10.1146/annurev.cellbio.20.012103.094623?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed.
External links
- Eukaryotic Linear Motif resource motif class LIG_Dynein_DLC8_1
- The Dynein Homepage
- Ron Vale's iBioSeminar on Motor Proteins
- MeSH Dynein
- EC 3.6.4.2
Proteins of the cytoskeleton Human I (MYO1A, MYO1B, MYO1C, MYO1D, MYO1E, MYO1F, MYO1G, MYO1H) · II (MYH1, MYH2, MYH3, MYH4, MYH6, MYH7, MYH7B, MYH8, MYH9, MYH10, MYH11, MYH13, MYH14, MYH15, MYH16) · III (MYO3A, MYO3B) · V (MYO5A, MYO5B, MYO5C) · VI (MYO6) · VII (MYO7A, MYO7B) · IX (MYO9A, MYO9B) · X (MYO10) · XV (MYO15A) · XVIII (MYO18A, MYO18B) · LC (MYL1, MYL2, MYL3, MYL4, MYL5, MYL6, MYL6B, MYL7, MYL9, MYLIP, MYLK, MYLK2, MYLL1)OtherOtherEpithelial keratins
(soft alpha-keratins)Ungrouped alphaNot alphaType 3Type 4Type 5DyneinsOtherOtherNonhuman Hydrolases: acid anhydride hydrolases (EC 3.6) 3.6.1 3.6.2 3.6.3-4: ATPase 3.6.3Cu++ (3.6.3.4)ATP4AOther P-type ATPase3.6.43.6.5: GTPase 3.6.5.3: Protein-synthesizing GTPaseCategories:- Motor proteins
Wikimedia Foundation. 2010.