- Retina
-
Retina 
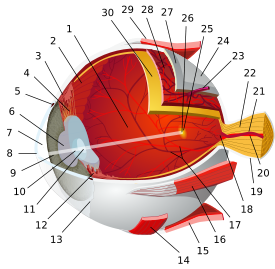
Right human eye cross-sectional view. Courtesy NIH National Eye Institute. Many animals have eyes different from the human eye. Gray's subject #225 1014 Artery central retinal artery MeSH Retina Dorlands/Elsevier Retina The vertebrate retina (from Latin rēte, meaning "net") is a light-sensitive tissue lining the inner surface of the eye. The optics of the eye create an image of the visual world on the retina, which serves much the same function as the film in a camera. Light striking the retina initiates a cascade of chemical and electrical events that ultimately trigger nerve impulses. These are sent to various visual centers of the brain through the fibers of the optic nerve.
In vertebrate embryonic development, the retina and the optic nerve originate as outgrowths of the developing brain, so the retina is considered part of the central nervous system (CNS).[1] It is the only part of the CNS that can be visualized non-invasively.
The retina is a layered structure with several layers of neurons interconnected by synapses. The only neurons that are directly sensitive to light are the photoreceptor cells. These are mainly of two types: the rods and cones. Rods function mainly in dim light and provide black-and-white vision, while cones support daytime vision and the perception of colour. A third, much rarer type of photoreceptor, the photosensitive ganglion cell, is important for reflexive responses to bright daylight.
Neural signals from the rods and cones undergo processing by other neurons of the retina. The output takes the form of action potentials in retinal ganglion cells whose axons form the optic nerve. Several important features of visual perception can be traced to the retinal encoding and processing of light.
Contents
Invertebrate retinae
The retinae of a jumping spider's main eyes, set in the front-and-centre of the cephalothorax and forming tubes extending well into the cephalothorax, are more acute in daylight than a cat's and 10 times more acute than a dragonfly's.[2] [3]
Anatomy of vertebrate retina
The vertebrate retina has ten distinct layers.[4] From closest to farthest from the vitreous body - that is, from closest to the front exterior of the head towards the interior and back of the head:
- Inner limiting membrane – Müller cell footplates
- Nerve fiber layer – axons of the ganglion cell nuclei
- Ganglion cell layer – contains nuclei of ganglion cells, the axons of which become the optic nerve fibers for messages.[1]
- Inner plexiform layer – contains the synapse between the bipolar cell axons and the dendrites of the ganglion and amacrine cells.[1]
- Inner nuclear layer – contains the nuclei and surrounding cell bodies (perikarya) of the bipolar cells.[1]
- Outer plexiform layer – projections of rods and cones ending in the rod spherule and cone pedicle, respectively. These make synapses with dendrites of bipolar[1] In the macular region, this is known as the Fiber layer of Henle.
- Outer nuclear layer – cell bodies of rods and cones
- External limiting membrane – layer that separates the inner segment portions of the photoreceptors from their cell nucleus
- Photoreceptor layer – rods/cones
- Retinal pigment epithelium - single layer of cuboidal cells
These can be simplified into 4 main processing stages: photoreception, transmission to bipolar cells, transmission to ganglion cells which also contain photoreceptors, the photosensitive ganglion cells, and transmission along the optic nerve. At each synaptic stage there are also laterally connecting horizontal and amacrine cells.
The optic nerve is a central tract of many axons of ganglion cells connecting primarily to the lateral geniculate body, a visual relay station in the diencephalon (the rear of the forebrain). It also projects to the superior colliculus, the suprachiasmatic nucleus, and the nucleus of the optic tract. It passes through the other layers creating the Optic disc in primates.[5]
Additional structures, not directly associated with vision, are found as outgrowths of the retina in some vertebrate groups. In birds, the pecten is a vascular structure of complex shape that projects from the retina into the vitreous humour; it supplies oxygen and nutrients to the eye, and may also aid in vision. Reptiles have a similar, but much simpler, structure, referred to as the papillary cone.[6]
Physical structure of human retina
In adult humans, the entire retina is approximately 72% of a sphere about 22 mm in diameter. The entire retina contains about 7 million cones and 75 to 150 million rods. The optic disc, a part of the retina sometimes called "the blind spot" because it lacks photoreceptors, is located at the optic papilla, a nasal zone where the optic-nerve fibers leave the eye. It appears as an oval white area of 3mm². Temporal (in the direction of the temples) to this disc is the macula. At its center is the fovea, a pit that is responsible for our sharp central vision but is actually less sensitive to light because of its lack of rods. Human and non-human primates possess one fovea as opposed to certain bird species such as hawks who actually are bifoviate and dogs and cats who possess no fovea but a central band known as the visual streak. Around the fovea extends the central retina for about 6 mm and then the peripheral retina. The edge of the retina is defined by the ora serrata. The length from one ora to the other (or macula), the most sensitive area along the horizontal meridian is about 3.2 mm.
 Rods, cones and nerve layers in the retina. The front (anterior) of the eye is on the left. Light (from the left) passes through several transparent nerve layers to reach the rods and cones (far right). A chemical change in the rods and cones send a signal back to the nerves. The signal goes first to the bipolar and horizontal cells (yellow layer), then to the amacrine cells and ganglion cells (purple layer), then to the optic nerve fibers. The signals are processed in these layers. First, the signals start as raw outputs of points in the rod and cone cells. Then the nerve layers identify simple shapes, such as bright points surrounded by dark points, edges, and movement. (Based on a drawing by Ramón y Cajal.)
Rods, cones and nerve layers in the retina. The front (anterior) of the eye is on the left. Light (from the left) passes through several transparent nerve layers to reach the rods and cones (far right). A chemical change in the rods and cones send a signal back to the nerves. The signal goes first to the bipolar and horizontal cells (yellow layer), then to the amacrine cells and ganglion cells (purple layer), then to the optic nerve fibers. The signals are processed in these layers. First, the signals start as raw outputs of points in the rod and cone cells. Then the nerve layers identify simple shapes, such as bright points surrounded by dark points, edges, and movement. (Based on a drawing by Ramón y Cajal.)
In section the retina is no more than 0.5 mm thick. It has three layers of nerve cells and two of synapses, including the unique ribbon synapses. The optic nerve carries the ganglion cell axons to the brain and the blood vessels that open into the retina. The ganglion cells lie innermost in the retina while the photoreceptive cells lie outermost. Because of this counter-intuitive arrangement, light must first pass through and around the ganglion cells and through the thickness of the retina, (including its capillary vessels, not shown) before reaching the rods and cones. However it does not pass through the epithelium or the choroid (both of which are opaque).
The white blood cells in the capillaries in front of the photoreceptors can be perceived as tiny bright moving dots when looking into blue light. This is known as the blue field entoptic phenomenon (or Scheerer's phenomenon).
Between the ganglion cell layer and the rods and cones there are two layers of neuropils where synaptic contacts are made. The neuropil layers are the outer plexiform layer and the inner plexiform layer. In the outer the rods and cones connect to the vertically running bipolar cells, and the horizontally oriented horizontal cells connect to ganglion cells.
The central retina is cone-dominated and the peripheral retina is rod-dominated. In total there are about seven million cones and a hundred million rods. At the centre of the macula is the foveal pit where the cones are smallest and in a hexagonal mosaic, the most efficient and highest density. Below the pit the other retina layers are displaced, before building up along the foveal slope until the rim of the fovea or parafovea which is the thickest portion of the retina. The macula has a yellow pigmentation from screening pigments and is known as the macula lutea. The area directly surrounding the fovea has the highest density of rods converging on single bipolars. Since the cones have a much lesser power of merging signals, the fovea allows for the sharpest vision the eye can attain.[1]
Though the rod and cones are a mosaic of sorts, transmission from receptors to bipolars to ganglion cells is not the case, Since there are about 150 million receptors and only 1 million optic nerve fibers, there must be convergence and thus mixing of signals. Moreover, the horizontal action of the horizontal and amacrine cells can allow one area of the retina to control another (e.g., one stimulus inhibiting another). This inhibition is key to the sum of messages sent to the higher regions of the brain. In some lower vertebrates, (e.g., the pigeon) there is a "centrifugal" control of messages, that is, one layer can control another, or higher regions of the brain can drive the retinal nerve cells, but in primates this does not occur.[1]
Vertebrate and cephalopod retina differences
The vertebrate retina is inverted in the sense that the light sensing cells sit at the back side of the retina, so that light has to pass through layers of neurons and capillaries before it reaches the rods and cones. By contrast, the cephalopod retina has the photoreceptors at the front side of the retina, with processing neurons and capillaries behind them. Because of this, cephalopods do not have a blind spot.
The cephalopod retina does not originate as an outgrowth of the brain, as the vertebrate one does. It was originally argued that this difference shows that vertebrate and cephalopod eyes are not homologous but have evolved separately. The evolutionary biologist Richard Dawkins cites the imperfect structure of the human retina as confounding claims by creationists or intelligent design theorists that the human eye is so perfect it must have a designer.[7]
In 2009 Kröger anatomically showed in Zebrafish that though the inverted arrangement is nonadaptive in that it creates avoidable scattering of light (and thus loss of light and image blur), it has space-saving advantages for small-eyed animals in which there is a minimal vitreous body, as the space between the lens and the photoreceptors’ light-sensitive outer segments is completely filled with retinal cells.[8]
The difference between vertebrate and cephalopod retinas presents an interesting puzzle of evolutionary path which is not yet fully settled. From an evolutionary perspective, a convoluted structure such as the inverted retina can generally come about as a consequence of two alternative processes; (a) an advantageous "good" compromise between competing functional limitations, or (b) as a historical maladaptive relic of the convoluted path of organ evolution and transformation. Vision is an important adaptation in higher vertebrates. Therefore, if the retina is indeed “wired wrongly” or “badly designed” (from an optical engineering point of view) then it sensible to look for it to possibly have some very significant physiological advantage. One such suggestion is based on the argument that the mammalian photoreceptor amplification process requires vast quantities of metabolic energy, and consequently, it requires massive and homogeneous supply of blood. Indeed, a unique network of blood vessels gives impression of being well adapted to provide the photoreceptor layer with copious quantities of blood. This lead to a proposition that the inverted retina is an adaptation to deliver abundant quantities of oxygen to the photoreceptor cells commensurate with their high energy demands.
Yet, the cephalopods have a non-inverted retina which is comparable in resolving power to the eyes of many vertebrates. Hence, at least for cold-blooded vertebrates, the inverted retinal structure is almost certainly not an adaptive necessity. All together, the inverted retina structure remains a mystery. Why should such an unlikely arrangement have appeared in the first place some 600 million years ago in the earliest of vertebrates who had presumably no need for high acuity vision and in all probability possessed photoreceptors with lower metabolic rates than those of higher warm-blooded vertebrates today? If the non-inverted retina works so well for the cold-blooded cephalopods, why did evolution go down the path of having inverted retina in most cold-blooded vertebrates (e.g., fish)? We are likely to be still missing a satisfactory scientific understanding of either (a) some hidden optimal physiological advantages of the inverted retina structure, or (b) a well-reasoned evolutionary path of how the retina has evolved in early stages from some other organ or structures so that the inverted retina is a sub-optimal relic of the evolutionary path. E.g., early in the Evolution of the eye, there was a cup-shaped hollow, with the vertebrate retina represented by a single cell layer that lined the eye's cavity. This layer then became increasingly photosensitive, evolving into rods and cones. Later, this single layer would be supplemented by additional neurons, creating cross-connections for logic processing and encoding of signals. In the primitive eye, there was no lens or vitreous body. Added layers then likely came to be positioned "on top" of older structures in the bottom of the cup, finally ending up between the rods and cones and the vitreous body.Physiology
An image is produced by the patterned excitation of the cones and rods in the retina. The excitation is processed by the neuronal system and various parts of the brain working in parallel to form a representation of the external environment in the brain.
The cones respond to bright light and mediate high-resolution colour vision during daylight illumination (also called photopic vision). The rods are saturated at daylight levels and don't contribute to pattern vision. However, rods do respond to dim light and mediate lower-resolution, monochromatic vision under very low levels of illumination (called scotopic vision). The illumination in most office settings falls between these two levels and is called mesopic vision. At these light levels, both the rods and cones are actively contributing pattern information to that exiting the eye. What contribution the rod information makes to pattern vision under these circumstances is unclear.
The response of cones to various wavelengths of light is called their spectral sensitivity. In normal human vision, the spectral sensitivity of a cone falls into one of three subgroups. These are often called blue, green, and red cones but more accurately are short, medium, and long wavelength sensitive cone subgroups. It is a lack of one or more of the cone subtypes that causes individuals to have deficiencies in colour vision or various kinds of colour blindness. These individuals are not blind to objects of a particular colour but experience the inability to distinguish between two groups of colours that can be distinguished by people with normal vision. Humans have three different types of cones (trichromatic vision) while most other mammals lack cones with red sensitive pigment and therefore have poorer (dichromatic) colour vision. However, some animals have four spectral subgroups, e.g., the trout adds an ultraviolet subgroup to short, medium and long subgroups that are similar to humans. Some fish are sensitive to the polarization of light as well.
When light falls on a receptor it sends a proportional response synaptically to bipolar cells which in turn signal the retinal ganglion cells. The receptors are also 'cross-linked' by horizontal cells and amacrine cells, which modify the synaptic signal before the ganglion cells. Rod and cone signals are intermixed and combine, although rods are mostly active in very poorly lit conditions and saturate in broad daylight, while cones function in brighter lighting because they are not sensitive enough to work at very low light levels.
Despite the fact that all are nerve cells, only the retinal ganglion cells and few amacrine cells create action potentials. In the photoreceptors, exposure to light hyperpolarizes the membrane in a series of graded shifts. The outer cell segment contains a photopigment. Inside the cell the normal levels of cyclic guanosine monophosphate (cGMP) keep the Na+ channel open and thus in the resting state the cell is depolarised. The photon causes the retinal bound to the receptor protein to isomerise to trans-retinal. This causes receptor to activate multiple G-proteins. This in turn causes the Ga-subunit of the protein to activate a phosphodiesterase (PDE6), which degrades cGMP, resulting in the closing of Na+ cyclic nucleotide-gated ion channels (CNGs). Thus the cell is hyperpolarised. The amount of neurotransmitter released is reduced in bright light and increases as light levels fall. The actual photopigment is bleached away in bright light and only replaced as a chemical process, so in a transition from bright light to darkness the eye can take up to thirty minutes to reach full sensitivity (see Adaptation (eye)).
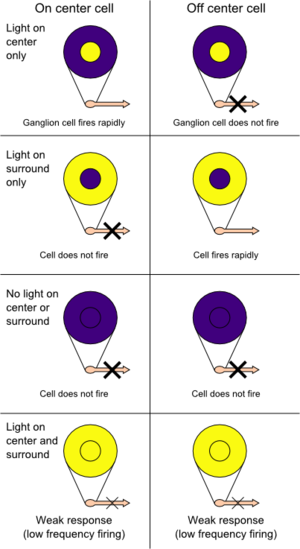
In the retinal ganglion cells there are two types of response, depending on the receptive field of the cell. The receptive fields of retinal ganglion cells comprise a central approximately circular area, where light has one effect on the firing of the cell, and an annular surround, where light has the opposite effect on the firing of the cell. In ON cells, an increment in light intensity in the centre of the receptive field causes the firing rate to increase. In OFF cells, it makes it decrease. In a linear model, this response profile is well described by a Difference of Gaussians and is the basis for edge detection algorithms. Beyond this simple difference ganglion cells are also differentiated by chromatic sensitivity and the type of spatial summation. Cells showing linear spatial summation are termed X cells (also called parvocellular, P, or midget ganglion cells), and those showing non-linear summation are Y cells (also called magnocellular, M, or parasol retinal ganglion cells), although the correspondence between X and Y cells (in the cat retina) and P and M cells (in the primate retina) is not as simple as it once seemed.
In the transfer of visual signals to the brain, the visual pathway, the retina is vertically divided in two, a temporal (nearer to the temple) half and a nasal (nearer to the nose) half. The axons from the nasal half cross the brain at the optic chiasma to join with axons from the temporal half of the other eye before passing into the lateral geniculate body.
Although there are more than 130 million retinal receptors, there are only approximately 1.2 million fibres (axons) in the optic nerve; a large amount of pre-processing is performed within the retina. The fovea produces the most accurate information. Despite occupying about 0.01% of the visual field (less than 2° of visual angle), about 10% of axons in the optic nerve are devoted to the fovea. The resolution limit of the fovea has been determined at around 10,000 points. The information capacity is estimated at 500,000 bits per second (for more information on bits, see information theory) without colour or around 600,000 bits per second including colour[citation needed].
Spatial encoding
The retina, unlike a camera, does not simply send a picture to the brain. The retina spatially encodes (compresses) the image to fit the limited capacity of the optic nerve. Compression is necessary because there are 100 times more Photoreceptor cells than ganglion cells as mentioned above. The retina does so by "decorrelating" the incoming images in a manner to be described below. These operations are carried out by the center surround structures as implemented by the bipolar and ganglion cells.
There are two types of center surround structures in the retina—on-centers and off-centers. On-centers have a positively weighted center and a negatively weighted surround. Off-centers are just the opposite. Positive weighting is more commonly known as excitatory and negative weighting is more commonly known as inhibitory.
These center surround structures are not physical in the sense that one cannot see them by staining samples of tissue and examining the retina's anatomy. The center surround structures are logical (i.e., mathematically abstract) in the sense that they depend on the connection strengths between ganglion and bipolar cells. It is believed that the connection strengths between cells is caused by the number and types of ion channels embedded in the synapses between the ganglion and bipolar cells. Stephen Kuffler in the 1950s was the first person to begin to understand these center surround structures in the retina of cats. See Receptive field for figures and more information on center surround structures. See chapter 3 of David Hubel's on-line book (listed below) for an excellent introduction.
The center surround structures are mathematically equivalent to the edge detection algorithms used by computer programmers to extract or enhance the edges in a digital photograph. Thus the retina performs operations on the image to enhance the edges of objects within its visual field. For example, in a picture of a dog, a cat and a car, it is the edges of these objects that contain the most information. In order for higher functions in the brain (or in a computer for that matter) to extract and classify objects such as a dog and a cat, the retina is the first step to separating out the various objects within the scene.
As an example, the following matrix is at the heart of the computer algorithm that implements edge detection. This matrix is the computer equivalent to the center surround structure. In this example, each box (element) within this matrix would be connected to one photoreceptor. The photoreceptor in the center is the current receptor being processed. The center photoreceptor is multiplied by the +1 weight factor. The surrounding photoreceptors are the "nearest neighbors" to the center and are multiplied by the -1/8 value. The sum of all nine of these elements is finally calculated. This summation is repeated for every photoreceptor in the image by shifting left to the end of a row and then down to the next line.
-1/8 -1/8 -1/8 -1/8 +1 -1/8 -1/8 -1/8 -1/8 The total sum of this matrix is zero if all the inputs from the nine photoreceptors are the same value. The zero result indicates the image was uniform (non-changing) within this small patch. Negative or positive sums mean something was varying (changing) within this small patch of nine photoreceptors.
The above matrix is only an approximation to what really happens inside the retina. The differences are:
- The above example is called "balanced". The term balanced means that the sum of the negative weights is equal to the sum of the positive weights so that they cancel out perfectly. Retinal ganglion cells are almost never perfectly balanced.
- The table is square while the center surround structures in the retina are circular.
- Neurons operate on spike trains traveling down nerve cell axons. Computers operate on a single Floating point number that is essentially constant from each input pixel. (The computer pixel is basically the equivalent of a biological photoreceptor.)
- The retina performs all these calculations in parallel while the computer operates on each pixel one at a time. There are no repeated summations and shifting as there would be in a computer.
- Finally, the horizontal and amacrine cells play a significant role in this process but that is not represented here.
Here is an example of an input image and how edge detection would modify it.

Once the image is spatially encoded by the center surround structures, the signal is sent out the optical nerve (via the axons of the ganglion cells) through the optic chiasm to the LGN (lateral geniculate nucleus). The exact function of the LGN is unknown at this time. The output of the LGN is then sent to the back of the brain. Specifically the output of the LGN "radiates" out to the V1 Primary visual cortex.
Simplified Signal Flow: Photoreceptors → Bipolar → Ganglion → Chiasm → LGN → V1 cortex
Diseases and disorders
Further information: List of eye diseases and disordersThere are many inherited and acquired diseases or disorders that may affect the retina. Some of them include:
- Retinitis pigmentosa is a group of genetic diseases that affect the retina and causes the loss of night vision and peripheral vision.
- Macular degeneration describes a group of diseases characterized by loss of central vision because of death or impairment of the cells in the macula.
- Cone-rod dystrophy (CORD) describes a number of diseases where vision loss is caused by deterioration of the cones and/or rods in the retina.
- In retinal separation, the retina detaches from the back of the eyeball. Ignipuncture is an outdated treatment method. The term retinal detachment is used to describe a separation of the neurosensory retina from the retinal pigment epithelium.[9] There are several modern treatment methods for fixing a retinal detachment: pneumatic retinopexy, scleral buckle, cryotherapy, laser photocoagulation and pars plana vitrectomy.
- Both hypertension and diabetes mellitus can cause damage to the tiny blood vessels that supply the retina, leading to hypertensive retinopathy and diabetic retinopathy.
- Retinoblastoma is a cancer of the retina.
- Retinal diseases in dogs include retinal dysplasia, progressive retinal atrophy, and sudden acquired retinal degeneration.
- Lipemia retinalis is a white appearance of the retina, and can occur by lipid deposition in lipoprotein lipase deficiency.
Diagnosis and treatment
A number of different instruments are available for the diagnosis of diseases and disorders affecting the retina. An ophthalmoscope is used to examine the retina. Recently, adaptive optics has been used to image individual rods and cones in the living human retina and a company based in Scotland have engineered technology that allows physicians to observe the complete retina without any discomfort to patients.[10]
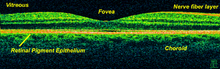
The electroretinogram is used to measure non-invasively the retina's electrical activity, which is affected by certain diseases. A relatively new technology, now becoming widely available, is optical coherence tomography (OCT). This non-invasive technique allows one to obtain a 3D volumetric or high resolution cross-sectional tomogram of the retinal fine structure with histologic-quality.
Treatment depends upon the nature of the disease or disorder. Transplantation of retinas has been attempted, but without much success. At MIT, The University of Southern California, and the University of New South Wales, an "artificial retina" is under development: an implant which will bypass the photoreceptors of the retina and stimulate the attached nerve cells directly, with signals from a digital camera.
Retinal blood supply
There are two circulations, both supplied by the ophthalmic artery. The uveal circulation consists of arteries entering the globe outside the optic nerve, these supply the uvea and outer and middle layers of the retina. The retinal circulation, on the other hand, supplies the inner layer of the retina and passes with the optic nerve as a branch of the ophthalmic artery called the central artery of the retina.[1] The unique structure of the blood vessels in the retina has been used for biometric identification.
The vascular topographical geometry in the retina is known to conform to structural principles that are related to certain physical properties [11]. The analysis of the geometrical structure is very important as deviations from the optimal principles may indicate some cardiovascular diseases, such as hypertension [12] and atherosclerosis [13]; a comprehensive analysis is given by Patton et al (2006) [14]. The identification of vascular bifurcations is one of the basic steps in this analysis. Azzopardi and Petkov (2011) [15] propose a computer vision algorithm that automatically detects these retinal features. Their results are evaluated against the ground truth data of vascular bifurcations of retinal fundus images that are obtained from the DRIVE data set.
Research
George Wald, Haldan Keffer Hartline and Ragnar Granit won the 1967 Nobel Prize in Physiology or Medicine for their scientific research on the retina.[16]
A recent University of Pennsylvania study calculated the approximate bandwidth of human retinas is 8.75 megabits per second, whereas guinea pig retinas transfer at 875 kilobits.[17]
MacLaren & Pearson and colleagues at University College London and Moorfields Eye Hospital in London showed in 2006 that photoreceptor cells could be transplanted successfully in the mouse retina if donor cells were at a critical developmental stage.[18] Recently Ader and colleagues in Dublin showed using the electron microscope that transplanted photoreceptors formed synaptic connections.[19]
Retinal gene therapy
Gene therapy holds promise as a potential avenue to cure a wide range of retinal diseases. This involves using a non-infectious virus to shuttle a gene into a part of the retina. Recombinant adeno-associated virus (rAAV) vectors possess a number of features that render them ideally suited for retinal gene therapy, including a lack of pathogenicity, minimal immunogenicity, and the ability to transduce postmitotic cells in a stable and efficient manner.[20] rAAV vectors are increasingly utilized for their ability to mediate efficient transduction of retinal pigment epithelium (RPE), photoreceptor cells and retinal ganglion cells. Each cell type can be specifically targeted by choosing the appropriate combination of AAV serotype, promoter, and intraocular injection site.
The unique architecture of the retina and its relatively immune-privileged environment help this process. Tight junctions that form the blood retinal barrier separate the subretinal space from the blood supply, thus protecting it from microbes and most immune-mediated damage, and enhancing its potential to respond to vector-mediated therapies. The highly compartmentalized anatomy of the eye facilitates accurate delivery of therapeutic vector suspensions to specific tissues under direct visualization using microsurgical techniques.[21] In the sheltered environment of the retina, AAV vectors are able to maintain high levels of transgene expression in the retinal pigmented epithelium (RPE), photoreceptors, or ganglion cells for long periods of time after a single treatment. In addition, the eye and the visual system can be routinely and easily monitored for visual function and retinal structural changes after injections with noninvasive advanced technology, such as visual acuities, contrast sensitivity, fundus auto-fluorescence (FAF), dark-adapted visual thresholds, vascular diameters, pupillometry, electroretinography (ERG), multifocal ERG and optical coherence tomography (OCT).[22]
This strategy is effective against retinal diseases that have been studied including neovascular diseases that are features of age-related macular degeneration, diabetic retinopathy and retinopathy of prematurity. Since the regulation of vascularization in the mature retina involves a balance between endogenous positive growth factors, such as vascular endothelial growth factor (VEGF) and inhibitors of angiogenesis, such as pigment epithelium-derived factor (PEDF), rAAV-mediated expression of PEDF, angiostatin, and the soluble VEGF receptor sFlt-1, which are all antiangiogenic proteins, have been shown to reduce aberrant vessel formation in animal models.[23] Since specific gene therapies cannot readily be used to treat a significant fraction of patients with retinal dystrophy, there is a major interest in developing a more generally applicable survival factor therapy. Neurotrophic factors have the ability to modulate neuronal growth during development to maintain existing cells and to allow recovery of injured neuronal populations in the eye. AAV encoding neurotrophic factors such as fibroblast growth factor (FGF) family members and GDNF either protected photoreceptors from apoptosis or slowed down cell death.[23]
However, treatment of inherited retinal degenerative diseases such as retinitis pigmentosa and Leber congenital amaurosis (LCA) via gene replacement therapy constitutes the most straightforward and therefore the most promising approach for treating the autosomal recessive retinal disease. Leber Congenital Amaurosis (LCA2) is a defect of the RPE65 gene, which is responsible for the synthesis of 11-cis retinal, an important molecule in the visual phototransduction, and gene replacement therapy studies utilizing rpe65-encoding AAV have yielded hopeful results in animal models.[24] Based on several encouraging reports from animal models, at least three clinical trials are currently underway for the treatment of LCA using modified AAV vectors carrying the RPE65 cDNA and have reported positive preliminary results.[25]
See also
- Adeno associated virus and gene therapy of the human retina
- Charles Schepens – "the father of modern retinal surgery"
- Evolution of the eye
- Duplex retina
- Retinal scan
References
- ^ a b c d e f g h "Sensory Reception: Human Vision: Structure and function of the Human Eye" vol. 27, Encyclopaedia Britannica, 1987
- ^ Harland, D.P., and Jackson, R.R. (2000). ""Eight-legged cats" and how they see: a review of recent research on jumping spiders (Araneae: Salticidae)" (PDF). Cimbebasia 16: 231–240. http://www.cogs.susx.ac.uk/ccnr/Papers/Downloads/Harland_Cimb2000.pdf. Retrieved 5 May 2011.
- ^ Hill, David Edwin (October 2010). "Use of location (relative direction and distance) information by jumping spiders (Araneae, Salticidae, Phidippus) during movement toward prey and other sighted objectives". Peckhamia 83 (1): 1–103. ISSN 1944-8120. http://peckhamia.com/peckhamia/PECKHAMIA%2083.1.pdf. Retrieved 12 April 2011.
- ^ The Retinal Tunic. Virginia-Maryland Regional College of Veterinary Medicine
- ^ Shepherd, Gordon (2004). The Synaptic Organization of the Brain. New York, NY: Oxford University Press. pp. 217–225. ISBN 978-0-19-515965-1.
- ^ Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. p. 465. ISBN 0-03-910284-X.
- ^ Dawkins, Richard (1986). The Blind Watchmaker. Longman. p. 93. ISBN 0-582-44694-5. "An engineer would laugh at any suggestion that the photocells might point away from the light, with their wires departing on the side nearest the light. Yet this is what happens in all vertebrate retinas. The wire has to travel over the surface of the retina to a point where it dives through a hole in the retina, (the so-called blind spot) to joint the optic nerve. Light..has to pass through a forest of connecting wires, presumably suffering at least some attenuation and distortion....the principle of the thing would offend any tidy-minded engineer"
- ^ Kröger RH, Biehlmaier O. (2009). Space saving advantage of an inverted retina. Vision Res. 49(18):2318-21. PMID 19591859
- ^ Oh, Kean, "Pathogenetic Mechanisms of Retinal Detachment", in Retina, ed. Ryan, S.J., Elsevier Health Sciences, Philadelphia, PA, 2006, p. 2013-2015
- ^ Seeing into the Future Ingenia, March 2007
- ^ Sherman, T: On connecting large vessels to small - the meaning of murray law. Journal of General Physiology vol. 78, pp. 431–453, 1981
- ^ Tso, M., Jampol, L.: Path-physiology of hypertensive retinopathy. Opthalmology vol. 89, 1982
- ^ Chapman, N., Dell’omo, G., Sartini, M.,Witt, N., Hughes, A., Thom, S., Pedrinelli, R.: Peripheral vascular disease is associated with abnormal arteriolar diameter relationships at bifurcations in the human retina. Clinical Science vol. 103, 2002
- ^ Patton, N., Aslam, T., MacGillivray, T., Deary, I., Dhillon, B., Eikelboom, R., Yogesan, K., Constable, I.: Retinal image analysis: Concepts, applications and potential. Progress in Retinal and Eye Research vol. 25, pp. 99–127, 2006
- ^ Azzopardi, G., Petkov, N.: Detection of retinal vascular bifurcations by trainable V4-like filters, in Computer Analysis of Images and Patterns (CAIP), Seville, Lecture Notes in Computer Science, vol. 6854, (Springer-Verlag Berlin Heidelberg, pp.451-459, 2011
- ^ The Nobel Prize in Physiology or Medicine 1967
- ^ Calculating the speed of sight - being-human - 28 July 2006 - New Scientist
- ^ Retinal repair by transplantation of photoreceptor precursors : Abstract : Nature
- ^ Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice
- ^ Astra Dinculescu, Lyudmyla Glushakova, Seok-Hong Min, William W. Hauswirth. Human Gene Therapy. June 2005, 16(6): 649-663.
- ^ Enrico M. Curace, Alberto Auricchio. Versatility of AAV vectors for retinal gene transfer. Vision Research. 2008, 48:353-359.
- ^ Anneke I. den Hollandera, Ronald Roepmana, Robert K. Koenekoopb, Frans P.M. Cremersa. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Progress in Retinal and Eye Research. July 2008; 27(4):391-419.
- ^ a b Rolling F. Recombinant AAV-mediated gene transfer to the retina: gene therapy perspectives. Gene Therapy. 2004; 11: S26-S32.
- ^ Tonia S. Rex. Rescue of Sight by Gene Therapy – Closer than It May Appear. Ophthalmic Genetics. 2007, 28:127-133.
- ^ Xue Cai, Shannon M. Conley, Muna I Naash. RPE65: Role in the Visual Cycle, Human Retinal Disease, and Gene Therapy. Ophthalmic Genetics. June 2009, 30(2):57-62.
Further reading
- S. Ramón y Cajal, Histologie du Système Nerveux de l'Homme et des Vertébrés, Maloine, Paris, 1911.
- Rodieck RW (1965). "Quantitative analysis of cat retinal ganglion cell response to visual stimuli". Vision Res. 5 (11): 583–601. doi:10.1016/0042-6989(65)90033-7. PMID 5862581.
- Wandell, Brian A. (1995). Foundations of vision. Sunderland, Mass: Sinauer Associates. ISBN 0-87893-853-2.
- Wässle H, Boycott BB (1991). "Functional architecture of the mammalian retina". Physiol Rev. 71 (2): 447–480. PMID 2006220.
- Schulz HL, Goetz T, Kaschkoetoe J, Weber BH (2004). "The Retinome – Defining a reference transcriptome of the adult mammalian retina/retinal pigment epithelium". BMC Genomics 5 (1): 50. doi:10.1186/1471-2164-5-50. PMC 512282. PMID 15283859. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=512282.
External links
- Eye, Brain, and Vision - online book - by David Hubel
- Kolb, H., Fernandez, E., & Nelson, R. (2003). Webvision: The neural organization of the vertebrate retina. Salt Lake City, Utah: John Moran Eye Center, University of Utah. Retrieved July 19, 2004.
- Demo: Artificial Retina, MIT Technology Review, September 2004. Reports on implant research at Technology Review
- Successful photoreceptor transplantation, MIT Technology Review, November 2006. How stem cells might restore sight Technology Review
- Australian Vision Prosthesis Group, Graduate School of Biomedical Engineering, University of New South Wales
- RetinaCentral, Genetics and Diseases of the Human Retina at University of Würzburg
- Retinal layers image. NeuroScience 2nd Ed at United States National Library of Medicine
- iBioSeminar: The Vertebrate Retina: Structure, Function, and Evolution on-line lecture by Jeremy Nathans
- Retina - Cell Centered Database
- Histology at BU 07901loa
Sensory system – visual system – globe of eye (TA A15.2.1–6, TH 3.11.08.0-5, GA 10.1005) Fibrous tunic (outer) Uvea/vascular tunic (middle) Retina (inner) LayersCellsPhotoreceptor cells (Cone cell, Rod cell) → (Horizontal cell) → Bipolar cell → (Amacrine cell) → Retina ganglion cell (Midget cell, Parasol cell, Bistratified cell, Giant retina ganglion cells, Photosensitive ganglion cell) → Diencephalon: P cell, M cell, K cell
Muller gliaOtherAnterior segment Posterior segment Other M: EYE
anat(g/a/p)/phys/devp/prot
noco/cong/tumr, epon
proc, drug(S1A/1E/1F/1L)
Categories:- Visual system
- Eye anatomy
Wikimedia Foundation. 2010.