- Dicloxacillin
-
Dicloxacillin 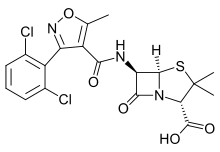
Systematic (IUPAC) name (2S,5R,6R)-6-{[3-(2,6-dichlorophenyl)-5-methyl-
oxazole-4-carbonyl]amino}-3,3-dimethyl-7-oxo-4-thia-
1-azabicyclo[3.2.0]heptane-2-carboxylic acidClinical data AHFS/Drugs.com monograph MedlinePlus a685017 Pregnancy cat. B2(AU) B(US) Legal status Prescription Only (S4) (AU) ℞-only (US) Routes Oral Pharmacokinetic data Bioavailability 60 to 80% Protein binding 98% Metabolism Hepatic Half-life 0.7 hours Excretion Renal and biliary Identifiers CAS number 3116-76-5 
ATC code J01CF01 QJ51CF01 PubChem CID 18381 DrugBank APRD00916 ChemSpider 17358 
UNII COF19H7WBK 
KEGG D02348 
ChEBI CHEBI:4511 
ChEMBL CHEMBL893 
Chemical data Formula C19H17Cl2N3O5S Mol. mass 470.327 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Dicloxacillin (INN) is a narrow-spectrum beta-lactam antibiotic of the penicillin class. It is used to treat infections caused by susceptible Gram-positive bacteria. It is active against beta-lactamase-producing organisms such as Staphylococcus aureus,[1] which would otherwise be resistant to most penicillins. It is very similar to flucloxacillin and these two agents are considered interchangeable. Dicloxacillin is available under a variety of trade names including Diclocil (BMS).
Contents
Mode of action
Main article: Beta-lactam antibioticLike other β-lactam antibiotics, dicloxacillin acts by inhibiting the synthesis of bacterial cell walls. It inhibits cross-linkage between the linear peptidoglycan polymer chains that make up a major component of the cell wall of Gram-positive bacteria.
Medicinal chemistry
Dicloxacillin is insensitive to beta-lactamase (also known as penicillinase) enzymes secreted by many penicillin-resistant bacteria. The presence of the isoxazolyl group on the side-chain of the penicillin nucleus facilitates the β-lactamase resistance, since they are relatively intolerant of side-chain steric hindrance. Thus, it is able to bind to penicillin-binding proteins (PBPs) and inhibit peptidoglycan crosslinking, but is not bound by or inactivated by β-lactamases.
Clinical use
Dicloxacillin is more acid-stable than many other penicillins and can be given orally, in addition to parenteral routes. However, like methicillin, it is less potent than benzylpenicillin against non-β-lactamase-producing Gram-positive bacteria.
Dicloxacillin has similar pharmacokinetics, antibacterial activity, and indications to flucloxacillin, and the two agents are considered interchangeable. It is believed to have lower incidence of severe hepatic adverse effects than flucloxacillin, but a higher incidence of renal adverse effects. (Rossi, 2006)
Available forms
Dicloxacillin is commercially available as the sodium salt dicloxacillin sodium in capsules (250 or 500 mg) and injections (powder for reconstitution, 500 and 1000 mg per vial).
Indications
Dicloxacillin is indicated for the treatment of infections caused by susceptible bacteria. Specific approved indications include: (Rossi, 2006)
- Staphylococcal skin infections and cellulitis – including impetigo, otitis externa, folliculitis, boils, carbuncles, and mastitis
- Pneumonia (adjunct)
- Osteomyelitis, septic arthritis, throat infections, streptococcus
- Septicaemia
- Empirical treatment for endocarditis
- Surgical prophylaxis
Precautions/contraindications
Dicloxacillin is contraindicated in those with a previous history of allergy to penicillins, cephalosporins or carbapenems. It should also not be used in the eye, or those with a history of cholestatic hepatitis associated with the use of dicloxacillin or flucloxacillin.
It should be used with caution in the elderly; patients with renal impairment, where a reduced dose is required; and those with hepatic impairment, due to the risk of cholestatic hepatitis. (Rossi, 2006)
Adverse effects
Common adverse drug reactions (ADRs) associated with the use of dicloxacillin include: diarrhoea, nausea, rash, urticaria, pain and inflammation at injection site, superinfection (including candidiasis), allergy, and transient increases in liver enzymes and bilirubin. (Rossi, 2006)
On rare occasions, cholestatic jaundice (also referred to as cholestatic hepatitis) has been associated with dicloxacillin therapy. The reaction may occur up to several weeks after treatment has stopped, and takes weeks to resolve. The estimated incidence is 1 in 15,000 exposures, and is more frequent in people >55 years, females, and those with treatment longer than 2 weeks. (Joint Formulary Committee, 2005; Rossi, 2006)
Resistance
Despite dicloxacillin's being insensitive to beta-lactamses, some organisms have developed resistance to it and other narrow-spectrum β-lactam antibiotics including methicillin. Such organisms include methicillin-resistant Staphylococcus aureus (MRSA).
See also
- Beta-lactam antibiotic
- Flucloxacillin
- Amoxicillin (formerly Beecham, now GlaxoSmithKline)
References
- ^ Miranda-Novales G, Leaños-Miranda BE, Vilchis-Pérez M, Solórzano-Santos F (2006). "In vitro activity effects of combinations of cephalothin, dicloxacillin, imipenem, vancomycin and amikacin against methicillin-resistant Staphylococcus spp. strains". Ann. Clin. Microbiol. Antimicrob. 5: 25. doi:10.1186/1476-0711-5-25. PMC 1617116. PMID 17034644. http://www.ann-clinmicrob.com/content/5//25.
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
Categories:- Beta-lactam antibiotics
- Enantiopure drugs
- Isoxazoles
- Organochlorides
Wikimedia Foundation. 2010.
