- Cefotaxime
-
Cefotaxime 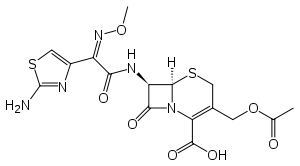
Systematic (IUPAC) name (6R,7R,Z)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-yl)-
2-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]
oct-2-ene-2-carboxylic acidClinical data Trade names Claforan AHFS/Drugs.com monograph MedlinePlus a682765 Pregnancy cat. B1(AU) B(US) Legal status Prescription Only (S4) (AU) Routes Intravenous Pharmacokinetic data Bioavailability n/a Metabolism Hepatic Half-life 0.8–1.4 hours Excretion 50–85% renal Identifiers CAS number 63527-52-6 ATC code J01DD01 PubChem CID 5479527 DrugBank APRD00854 ChemSpider 4586392 
UNII N2GI8B1GK7 
KEGG D07647 
ChEMBL CHEMBL102 
Chemical data Formula C16H17N5O7S2 Mol. mass 455.47 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Cefotaxime (INN) (
 /ˌsɛfɵˈtæksiːm/) is a third-generation cephalosporin antibiotic. Like other third-generation cephalosporins, it has broad spectrum activity against Gram positive and Gram negative bacteria. In most cases, it is considered to be equivalent to ceftriaxone in terms of safety and efficacy.
/ˌsɛfɵˈtæksiːm/) is a third-generation cephalosporin antibiotic. Like other third-generation cephalosporins, it has broad spectrum activity against Gram positive and Gram negative bacteria. In most cases, it is considered to be equivalent to ceftriaxone in terms of safety and efficacy.Contents
Mechanism of action
Inhibits bacterial cell wall synthesis by binding to one or more of the penicillin-binding proteins (PBPs) which in turn inhibits the final transpeptidation step of peptidoglycan synthesis in bacterial cell walls, thus inhibiting cell wall biosynthesis. Bacteria eventually lyse due to ongoing activity of cell wall autolytic enzymes (autolysins and murein hydrolases) while cell wall assembly is arrested.[1]
Cefotaxime, like other β-lactam antibiotics does not only block the division of bacteria, including cyanobacteria, but also the division of cyanelles, the photosynthetic organelles of the Glaucophytes, and the division of chloroplasts of bryophytes. In contrast, it has no effect on the plastids of the highly developed vascular plants. This is supporting the endosymbiotic theory and indicates an evolution of plastid division in land plants [2].
Clinical use
Cefotaxime is used for infections of the respiratory tract, skin, bones, joints, urogenital system, meningitis, and septicemia. It generally has good coverage against most Gram-negative bacteria, with the notable exception of Pseudomonas. It is also effective against most Gram-positive cocci except for Enterococcus.[1] It is active against penicillin-resistant strains of Streptococcus pneumoniae. It has modest activity against the anaerobic Bacteroides fragilis.
Chemistry
The syn-configuration of the methoxyimino moiety confers stability to β-lactamase enzymes produced by many Gram-negative bacteria. Such stability to β-lactamases increases the activity of cefotaxime against otherwise resistant Gram-negative organisms.
References
- ^ a b Cefotaxime drug information
- ^ Britta Kasten und Ralf Reski (1997): β-lactam antibiotics inhibit chloroplast division in a moss (Physcomitrella patens) but not in tomato (Lycopersicon esculentum). Journal of Plant Physiology 150, 137-140. [1]
Categories:- Cephalosporin antibiotics
- Thiazoles
- Oximes
- Acetate esters
Wikimedia Foundation. 2010.
