- Cefdinir
-
Cefdinir 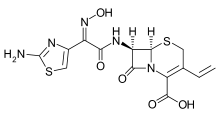
Systematic (IUPAC) name 8-[2-(2-amino-1,3-thiazol-4-yl)-1-hydroxy-2-nitroso-
ethenyl]amino-4-ethenyl-7-oxo-2-thia-6-
azabicyclo[4.2.0]oct-4-ene-5-carboxylic acidClinical data Trade names Omnicef AHFS/Drugs.com monograph MedlinePlus a698001 Pregnancy cat. B(US) Legal status ℞-only (US) Routes Oral Pharmacokinetic data Bioavailability 16% to 21% (dose-dependent) Protein binding 60% to 70% Metabolism Negligible Half-life 1.7 ± 0.6 hours Excretion Renal Identifiers CAS number 91832-40-5 ATC code J01DD15 PubChem CID 6915944 DrugBank APRD00644 ChemSpider 5291705 
UNII CI0FAO63WC 
KEGG D00917 
ChEBI CHEBI:3485 
ChEMBL CHEMBL927 
Chemical data Formula C14H13N5O5S2 Mol. mass 395.416 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Cefdinir (marketed by Abbott Laboratories under the brand name Omnicef) is a semi-synthetic, broad-spectrum antibiotic in the third generation of the cephalosporin class, proven effective for common bacterial infections of the ear, sinus, throat, and skin. It was approved by the U.S. Food and Drug Administration (FDA) in December 1997.
As of 2008, cefdinir was the highest-selling cephalosporin antibiotic in the United States, with more than US$585 million in retail sales of its generic versions alone.[1]
Contents
Mechanism of action
Indications
Therapeutic uses of cefdinir include otitis media, soft tissue infections, and respiratory tract infections, including sinusitis, strep throat, community-acquired pneumonia and acute exacerbations of bronchitis.
Susceptible organisms
Cefdinir is a bacteriocidal antibiotic. It can be used to treat infections caused by several Gram-negative and Gram-positive bacteria.
Side effects
According to the Omnicef website, side effects include "(...) diarrhea, vaginal infections or inflammation, nausea, headache, and abdominal pain."[2]
Available forms
Cefdinir is administered orally. It is available as capsules and a suspension. Dosage, schedule, and duration of therapy varies according to the type of infection.
Cefdinir is available as a generic drug.
"Blood" in the Stool
The pediatric version of Omnicef can bind to iron in the digestive tract. In rare cases, this creates a discoloration of the stool to a rust or red color. Some patients may interpret this as blood in the stool, although in reality blood appears dark brown or black in the stool. A doctor's office can perform a simple test on the stool to confirm that it does not have blood. If you simply wait one hour, the red color will remain red, (while real blood will turn black).
On the other hand, if the reddish stool is accompanied by abdominal pain, weight loss, diarrhea, etc., it could be a C. difficile (clostridium difficile) infection caused by the antibiotic. This infection can occur as a result of taking an antibiotic. Talk to your pediatrician immediately to see if you should stop using the antibiotic.
References
- ^ "2008 Top 200 generic drugs by retail dollars" PDF (399.4 KB). Drug Topics (May 26, 2009). Retrieved on July 24, 2009.
- ^ "Omnicef capsules Patient Information". Abbott Laboratories. February 2004. Archived from the original on 2006-11-18. http://web.archive.org/web/20061118132152/http://www.rxabbott.com/pdf/omnicef_capsules_patient_friendly.pdf. Retrieved 2006-11-24.
[1] U.S. Prescribing Information from Omnicef.com
Outside Links
Categories:- Cephalosporin antibiotics
- Thiazoles
Wikimedia Foundation. 2010.