- Mecillinam
-
Amdinocillin 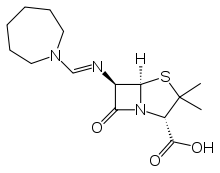
Systematic (IUPAC) name (2S,5R,6R)-6-[(azepan-1-ylmethylene)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. B(US)
Appears safe in pregnancy[1]Legal status ℞ Prescription only Routes Intravenous, intramuscular Pharmacokinetic data Bioavailability Negligible Protein binding 5 to 10% Metabolism Some hepatic metabolism Half-life 1 to 3 hours Excretion Renal and biliary, mostly unchanged Identifiers CAS number 32887-01-7 
ATC code J01CA11 PubChem CID 36273 DrugBank DB01163 ChemSpider 33357 
UNII V10579P3QZ 
KEGG D02888 
ChEMBL CHEMBL530 
Chemical data Formula C15H23N3O3S Mol. mass 325.426 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mecillinam (INN) or amdinocillin (USAN), trade name Coactin, is an extended-spectrum penicillin antibiotic that binds specifically to penicillin binding protein 2 (PBP2),[2] and is only considered to be active against Gram-negative bacteria. It is used primarily in the treatment of urinary tract infections, and has also been used to treat typhoid and paratyphoid fever.[3][4] Because mecillinam has very low oral bioavailability, an orally-active prodrug was developed: pivmecillinam. Neither drug is available in the United States.[5]
Contents
History
With the codename FL 1060, mecillinam was developed by the Danish pharmaceutical company Leo Pharmaceutical Products (now LEO Pharma). It was first described in the scientific literature in a 1972 paper.[6][7]
Activity
Mecillinam is active against most pathogenic Gram-negative bacteria, except Pseudomonas aeruginosa and some species of Proteus.[5] Several studies have also found it to be as effective as other antibiotics for treating Staphylococcus saprophyticus infection, even though it is Gram-positive, possibly because mecillinam reaches very high concentrations in urine.[1]
Worldwide resistance to mecillinam in bacteria causing urinary tract infection has remained very low since its introduction; a 2003 study conducted in 16 European countries and Canada found resistance to range between 1.2% (Escherichia coli) to 5.2% (Proteus mirabilis).[8] Another large study conducted in Europe and Brazil obtained similar results — 95.9% of E. coli strains, for instance, were sensitive to mecillinam.[9]
Adverse effects
See also: Beta-lactam antibiotic: Adverse effectsThe adverse effect profile of mecillinam is similar to that of other penicillins.[2] The most common side effects of mecillinam use are rash and gastrointestinal upset, including nausea and vomiting.[1]
References
- ^ a b c Nicolle LE (August 2000). = long&pmid = 10969050 "Pivmecillinam in the treatment of urinary tract infections". J Antimicrob Chemother 46 Suppl A (90001): 35–39. doi:10.1093/jac/46.suppl_1.35. PMID 10969050. http://jac.oxfordjournals.org/cgi/pmidlookup?view = long&pmid = 10969050.
- ^ a b Neu HC (1985). "Amdinocillin: a novel penicillin. Antibacterial activity, pharmacology and clinical use". Pharmacotherapy 5 (1): 1–10. PMID 3885172.
- ^ Clarke PD, Geddes AM, McGhie D, Wall JC (July 1976). "Mecillinam: a new antibiotic for enteric fever". Br Med J 2 (6026): 14–5. doi:10.1136/bmj.2.6026.14. PMC 1687648. PMID 820402. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1687648.
- ^ Geddes AM, Clarke PD (July 1977). "The treatment of enteric fever with mecillinam". J Antimicrob Chemother 3 Suppl B: 101–2. PMID 408321.
- ^ a b Pham P, Bartlett JG (August 28, 2008). "Amdinocillin (Mecillinam)". Point-of-Care Information Technology ABX Guide. Johns Hopkins University. http://prod.hopkins-abxguide.org/antibiotics/antibacterial/pcn_others/amdinocillin__mecillinam_.html. Retrieved on August 31, 2008. Freely available with registration.
- ^ Lund F, Tybring L (April 1972). "6β-amidinopenicillanic acids—a new group of antibiotics". Nature New Biol 236 (66): 135–7. doi:10.1038/236135c0. PMID 4402006.
- ^ Tybring L, Melchior NH (September 1975). "Mecillinam (FL 1060), a 6β-Amidinopenicillanic Acid Derivative: Bactericidal Action and Synergy In Vitro". Antimicrob Agents Chemother 8 (3): 271–6. PMC 429305. PMID 170856. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=170856.
- ^ Kahlmeter G (January 2003). "An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO·SENS Project". J Antimicrob Chemother 51 (1): 69–76. doi:10.1093/jac/dkg028. PMID 12493789. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=12493789.
- ^ Naber KG, Schito G, Botto H, Palou J, Mazzei T (May 2008). "Surveillance Study in Europe and Brazil on Clinical Aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): Implications for Empiric Therapy". Eur Urol 54 (5): 1164–75. doi:10.1016/j.eururo.2008.05.010. PMID 18511178.
Categories:- Beta-lactam antibiotics
- Enantiopure drugs
- Azepanes
Wikimedia Foundation. 2010.
