- Meropenem
-
Meropenem 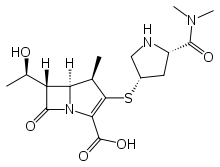
Systematic (IUPAC) name 3-[5-(dimethylcarbamoyl) pyrrolidin-2-yl] sulfanyl-6- (1-hydroxyethyl)-4-methyl-7-oxo- 1-azabicyclo[3.2.0] hept-2-ene-2-carboxylic acid Clinical data Trade names Merrem AHFS/Drugs.com monograph Pregnancy cat. B(US) Legal status POM (UK) ℞-only (US) Routes IV Pharmacokinetic data Bioavailability 100% Protein binding Approximately 2%. Half-life 1 hour Excretion Renal Identifiers CAS number 119478-56-7 
ATC code J01DH02 PubChem CID 441130 DrugBank APRD01097 ChemSpider 389924 
UNII FV9J3JU8B1 
KEGG D02222 
ChEBI CHEBI:43968 
ChEMBL CHEMBL127 
Chemical data Formula C17H25N3O5S Mol. mass 383.464 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Meropenem is an ultra-broad spectrum injectable antibiotic used to treat a wide variety of infections, including meningitis and pneumonia. It is a beta-lactam and belongs to the subgroup of carbapenem, similar to imipenem and ertapenem. Meropenem was originally developed by Sumitomo Pharmaceuticals. It is marketed outside Japan by AstraZeneca with the brand names Merrem and Meronem. It gained US FDA approval in July 1996. It penetrates well into many tissues and body fluids including the cerebrospinal fluid, bile, heart valves, lung, and peritoneal fluid.[1]
Contents
Mechanism of action
Meropenem is bactericidal except against Listeria monocytogenes where it is bacteriostatic. It inhibits bacterial wall synthesis like other beta-lactam antibiotics. In contrast to other beta-lactams, it is highly resistant to degradation by beta-lactamases or cephalosporinases. Resistance generally arises due to mutations in penicillin binding proteins, production of metallo-beta-lactamases, or resistance to diffusion across the bacterial outer membrane.[2] Unlike imipenem, it is stable to dehydropeptidase-1 and can therefore be given without cilastatin.
Indications
The spectrum of action includes many gram-positive and gram-negative bacteria (including Pseudomonas) and anaerobic bacteria. The overall spectrum is similar to imipenem although meropenem is more active against Enterobacteriaceae and less active against gram-positive bacteria. It is also very resistant to extended-spectrum beta lactamases but may be more susceptible to metallo-beta-lactamases.[1] Meropenem is frequently given in the treatment of febrile neutropenia. This condition frequently occurs in patients with hematological malignancies and cancer patients receiving anticancer drugs that cause bone marrow suppression..
Administration
Meropenem must be administered intravenously. It is supplied as a white crystalline powder to be dissolved in 5% monobasic potassium phosphate solution. Dosing must be adjusted for kidney function and for haemofiltration.[3]
Common adverse effects
The most common adverse effects are diarrhea (4.8%), nausea and vomiting (3.6%), injection-site inflammation (2.4%), headache (2.3%), rash (1.9%), and thrombophlebitis (0.9%).[2] Many of these adverse effects were observed in the setting of severely ill individuals who were already taking many medications. Meropenem also has a reduced potential for causing seizures in comparison with imipenem. Several cases of severe hypokalemia have been reported.[4][5]
Trade names
Other trade names include Zwipen, Carbonem and Neomed (India), Mepem (Taiwan), Meropen (Japan, Korea) and Neopenem. [Merocon (Continental), Carnem (Laderly Biotech), PENRO (Bosch), Meronem (ICI) {Pakistan}]
References
- ^ a b AHFS DRUG INFORMATION 2006 (2006 ed ed.). American Society of Health-System Pharmacists. 2006.
- ^ a b Mosby's Drug Consult 2006 (16 ed ed.). Mosby, Inc.. 2006.
- ^ Bilgrami, I.; Roberts, J. A.; Wallis, S. C.; Thomas, J.; Davis, J.; Fowler, S.; Goldrick, P. B.; Lipman, J. (2010). "Meropenem Dosing in Critically Ill Patients with Sepsis Receiving High Volume Continuous Veno-Venous Hemofiltration". Antimicrobial Agents and Chemotherapy 54 (7): 2974. doi:10.1128/AAC.01582-09. PMC 2897321. PMID 20479205. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2897321.
- ^ Margolin L. Impaired rehabilitation secondary to muscle weakness induced by meropenem. Clin. Drug Invest. 2004; 24(1):61-2.
- ^ R Bharti, S Gombar, AK Khanna. Meropenem in critical care - uncovering the truths behind weaning failure. J Anaesth Clin Pharmacol 2010; 26(1): 99-101
External links
- MERREM I.V. (meropenem for injection) Official Site by AstraZeneca
Categories:- Carbapenem antibiotics
- Enantiopure drugs
- Amides
- Pyrrolidines
Wikimedia Foundation. 2010.
