- Ceftazidime
-
Ceftazidime 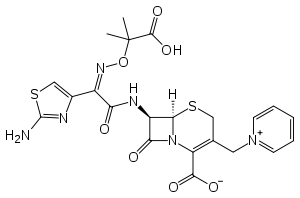
Systematic (IUPAC) name (6R,7R,Z)-7-(2-(2-aminothiazol-4-yl)-
2-(2-carboxypropan-2-yloxyimino)acetamido)-8-oxo-
3-(pyridinium-1-ylmethyl)-5-thia-1-aza-bicyclo[4.2.0]
oct-2-ene-2-carboxylateClinical data Trade names Fortaz, Tazicef AHFS/Drugs.com monograph MedlinePlus a686007 Pregnancy cat. B1(AU) B(US) Legal status Prescription Only (S4) (AU) Routes Intravenous, intramuscular Pharmacokinetic data Bioavailability 91% (IM) Metabolism negligible Half-life 1.6–2 hours Excretion 90–96% renal Identifiers CAS number 72558-82-8 ATC code J01DD02 PubChem CID 5481173 DrugBank APRD00857 ChemSpider 4587145 
UNII DZR1ENT301 
ChEBI CHEBI:3508 
ChEMBL CHEMBL201 
Chemical data Formula C22H22N6O7S2 Mol. mass 546.58 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ceftazidime (INN) (
 /sɛfˈtæzɨdiːm/) is a third-generation cephalosporin antibiotic. Like other third-generation cephalosporins, it has broad spectrum activity against Gram-positive and Gram-negative bacteria. Unlike most third-generation agents, it is active against Pseudomonas aeruginosa, however it has weaker activity against Gram-positive microorganisms and is not used for such infections. Ceftazidime pentahydrate is marketed under various trade names including Cefzim (Pharco B International), Fortum (GSK), and Fortaz.
/sɛfˈtæzɨdiːm/) is a third-generation cephalosporin antibiotic. Like other third-generation cephalosporins, it has broad spectrum activity against Gram-positive and Gram-negative bacteria. Unlike most third-generation agents, it is active against Pseudomonas aeruginosa, however it has weaker activity against Gram-positive microorganisms and is not used for such infections. Ceftazidime pentahydrate is marketed under various trade names including Cefzim (Pharco B International), Fortum (GSK), and Fortaz.Clinical use
Ceftazidime is usually reserved for the treatment of infections caused by Pseudomonas aeruginosa. It is also used in the empirical therapy of febrile neutropenia, in combination with other antibiotics. It is usually given IV or IM every 8–12 hours (2 - 3 times a day), with dosage varying by the indication, infection severity, and/or renal function of the recipient.
Ceftazidine is first line treatment for the rare tropical infection, melioidosis.[1]
Chemistry
In addition to the syn-configuration of the imino side chain, compared to other third-generation cephalosporins, the more complex moiety (containing two methyl and a carboxylic acid group) confers extra stability to β-lactamase enzymes produced by many Gram-negative bacteria. The extra stability to β-lactamases increases the activity of ceftazidime against otherwise resistant Gram-negative organisms including Pseudomonas aeruginosa. The charged pyridinum moiety increases water-solubility.
References
- ^ White NJ (2003). "Melioidosis". Lancet 361 (9370): 1715–722. doi:10.1016/S0140-6736(03)13374-0. PMID 12767750.
Categories:- Cephalosporin antibiotics
- Thiazoles
- Pyridines
- Zwitterions
Wikimedia Foundation. 2010.
