- Aztreonam
-
Aztreonam 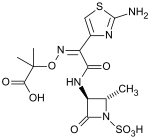
Systematic (IUPAC) name 2-({[(1Z)-1-(2-amino-1,3-thiazol-4-yl) -2- {[(2S,3S)-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino} -2- oxoethylidene]amino}oxy)-2-methylpropanoic acid Clinical data Trade names Azactam AHFS/Drugs.com monograph Pregnancy cat. B1 (Au), B (U.S.) Legal status ℞-only (U.S.) Routes Intravenous, intramuscular, inhalation Pharmacokinetic data Bioavailability 100% (IM) Protein binding 56% Metabolism hepatic (minor %) Half-life 1.7 hours Excretion Renal Identifiers CAS number 78110-38-0 
ATC code J01DF01 PubChem CID 54116 DrugBank APRD00815 ChemSpider 4674940 
UNII G2B4VE5GH8 
KEGG D00240 
ChEBI CHEBI:161680 
ChEMBL CHEMBL158 
Chemical data Formula C13H17N5O8S2 Mol. mass 435.433 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Aztreonam (trade names Azactam injection, Cayston inhalation) is a synthetic monocyclic beta-lactam antibiotic (a monobactam), with the nucleus based on a simpler monobactam isolated from Chromobacterium violaceum. It was approved by the U.S. Food and Drug Administration (FDA) in 1986. It is resistant to some beta-lactamases, but is inactivated by extended-spectrum beta-lactamases.
Contents
Mechanism of action
Aztreonam is similar in action to penicillin. It inhibits mucopeptide synthesis in the bacterial cell wall, thereby blocking peptidoglycan crosslinking. It has a very high affinity for penicillin-binding protein 3 (PBP-3) and mild affinity for PBP-1a. Aztreonam binds the penicillin-binding proteins of gram-positive and anaerobic bacteria very poorly and is largely ineffective against them.[1] Aztreonam is bactericidal but less so than some of the cephalosporins.
Indications and spectrum of activity
Aztreonam has strong activity against susceptible gram-negative bacteria, including Pseudomonas aeruginosa. It has no useful activity against gram-positive bacteria or anaerobes. It is known to be effective against a wide range of bacteria including Citrobacter, Enterobacter, E. coli, Haemophilus, Klebsiella, Proteus, and Serratia species.[2]
Synergism between aztreonam and arbekacin or tobramycin against Pseudomonas aeruginosa has been suggested.[3]
Aztreonam is often used in patients who are penicillin allergic or who can not tolerate aminoglycosides.
Administration
Aztreonam is poorly absorbed when given via the oral route, so it must be administered as an intravenous or intramuscular injection (trade name Azactam ), or inhaled (trade name Cayston) using an ultrasonic nebulizer. In the United States the FDA approved the inhalative form on February 22, 2010 for the suppression of Pseudomonas aeruginosa infections in patients with cystic fibrosis.[4] It received conditional approval for administration in Canada and the European Union September 2009,[4] and has been fully approved in Australia.[5]
Common adverse effects
Reported side-effects include injection site reactions, rash, and rarely toxic epidermal necrolysis. Gastrointestinal side effects generally include diarrhea and nausea and vomiting. There may be drug-induced eosinophilia. Because of the un-fused beta-lactam ring unique to aztreonam, there is limited cross-reactivity between aztreonam and other beta-lactam antibiotics, and it is generally considered safe to admininister aztreonam to patients with hypersensitivity (allergies) to penicillins.[1]
Aztreonam is considered Pregnancy category B.
References
- ^ a b AHFS DRUG INFORMATION 2006 (2006 ed.). American Society of Health-System Pharmacists. 2006.
- ^ Mosby's Drug Consult 2006 (16 ed.). Mosby, Inc.. 2006.
- ^ Kobayashi, Y., Uchida, H., Kawakami Y. (1992). "Synergy with aztreonam and arbekacin or tobramycin against Pseudomonas aeruginosa isolated from blood". J Antimicrob Chemother 30 (6): 871–872. doi:10.1093/jac/30.6.871. PMID 1289363.
- ^ a b Larkin, Catherine (February 22, 2010). "Gilead’s Inhaled Antibiotic for Lungs Wins Approval". BusinessWeek. http://www.businessweek.com/news/2010-02-22/gilead-s-inhaled-antibiotic-for-lungs-wins-approval-update1-.html. Retrieved 2010-03-05.
- ^ "FDA approves Gilead cystic fibrosis drug Cayston". BusinessWeek. February 23, 2010. http://www.businessweek.com/ap/financialnews/D9E237QG1.htm. Retrieved 2010-03-05.
External links
Categories:- Monobactam antibiotics
- Enantiopure drugs
- Thiazoles
- Azetidines
Wikimedia Foundation. 2010.
