- Benzylpenicillin
-
Penicillin G 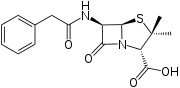
Systematic (IUPAC) name (2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information MedlinePlus a685013 Pregnancy cat. B(US) Legal status ℞ Prescription only Routes parenteral Pharmacokinetic data Protein binding 60 % Metabolism hepatic Half-life 30 min Excretion renal Identifiers CAS number 61-33-6  (free acid)
(free acid)
69-57-8 (sodium salt)ATC code J01CE01 S01AA14 QJ51CE01 PubChem CID 5904 DrugBank DB01053 ChemSpider 5693 
UNII Q42T66VG0C 
KEGG D02336 
ChEBI CHEBI:18208 
ChEMBL CHEMBL29 
Chemical data Formula C16H18N2O4S Mol. mass 334.4 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Benzylpenicillin, commonly known as penicillin G, is the gold standard type of penicillin. 'G' in the name 'Penicillin G' refers to 'Gold Standard'. Penicillin G is typically given by a parenteral route of administration (not orally) because it is unstable in the hydrochloric acid of the stomach. Because the drug is given parenterally, higher tissue concentrations of penicillin G can be achieved than is possible with phenoxymethylpenicillin. These higher concentrations translate to increased antibacterial activity.
Contents
Medical uses
Specific indications for benzylpenicillin include:[1]
- Cellulitis
- Bacterial endocarditis
- Gonorrhea
- Meningitis
- Aspiration pneumonia, lung abscess
- Community-acquired pneumonia
- Syphilis
- Septicemia in children
- Septic Arthritis
- Galactic Diarrhoea
- Gangrene
Adverse effects
Adverse affect can include hypersensitivity reactions including urticaria, fever, joint pains, rashes, angioedema, anaphylaxis, serum sickness-like reaction. Rarely CNS toxicity including convulsions (especially with high doses or in severe renal impairment), interstitial nephritis, haemolytic anaemia, leucopenia, thrombocytopenia, and coagulation disorders. Also reported diarrhoea (including antibiotic-associated colitis).
Toxicology
Benzylpenicillin serum concentrations can be monitored either by traditional microbiological assay or by more modern chromatographic techniques. Such measurements can be useful to avoid central nervous system toxicity in any patient receiving large doses of the drug on a chronic basis, but they are especially relevant to patients with renal failure, who may accumulate the drug due to reduced urinary excretion rates.[2][3]
Compendial status
References
- ^ Rossi S, editor, ed (2006). Australian Medicines Handbook. Adelaide: Australian Medicines Handbook. ISBN 0-9757919-2-3.
- ^ Fossieck B Jr, Parker RH. Neurotoxicity during intravenous infusion of penicillin. A review. J. Clin. Pharmacol. 14: 504- 512, 1974.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1195-1196.
- ^ British Pharmacopoeia Commission Secretariat. "Index (BP 2009)". http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf. Retrieved 26 March 2010.
Categories:- Beta-lactam antibiotics
- Enantiopure drugs
- World Health Organization essential medicines
Wikimedia Foundation. 2010.
