- Cefovecin
-
Cefovecin 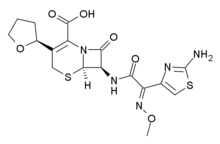
Systematic (IUPAC) name (7R)-7-([(2Z)- 2-(2-amino- 1,3-thiazol- 4-yl)- 2-methoxyiminoacetyl]amino)- 8-oxo- 3-[(2S)-oxolan- 2-yl]- 5-thia- 1-azabicyclo[4.2.0]oct- 2-ene- 2-carboxylic acid Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. do not administer to pregnant cat or during breeding or lactation period. Legal status veterinary prescription only Routes subcutaneous Pharmacokinetic data Metabolism non metabolised by the liver Half-life half life ellimination in cats is 7 days Excretion mostly excreted unchanged in the urine. Identifiers CAS number 234096-34-5 ATCvet code QJ01DD91 PubChem CID 9578573 UNII 0D1OL46ZIE 
Chemical data Formula C17H19N5O6S2 Mol. mass 453.49 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Cefovecin (INN) is an antibiotic of the cephalosporin class, licensed for the treatment of skin infections in cats and dogs. It is marketed by Pfizer under the trade name Convenia.
Approval and usage
Cefovecin was first authorized for use in the European Union on June 2006.[1] It was not approved for use in the United States until June 2008.[2]
References
- ^ "European Public Assessment Report for Convenia (from the EMEA website)". http://www.emea.europa.eu/vetdocs/vets/Epar/convenia/convenia.htm. Retrieved 2008-07-11.
- ^ "FDA Approves First and Only Single-Dose Antibiotic for Dogs and Cats" (Press release). Pfizer Inc.. 2008-06-30. http://www.businesswire.com/portal/site/google/?ndmViewId=news_view&newsId=20080630005686&newsLang=en. Retrieved 2008-07-11.
External links
- PubChem 23670874 - Cefovecin sodium
- Official website by Pfizer

This systemic antibacterial-related article is a stub. You can help Wikipedia by expanding it. This veterinary medicine–related article is a stub. You can help Wikipedia by expanding it.
