- Electric double-layer capacitor
-
An electric double-layer capacitor (EDLC), also known as supercapacitor, supercondenser, electrochemical double layer capacitor, or ultracapacitor, is an electrochemical capacitor with relatively high energy density. Their energy density is typically hundreds of times greater than conventional electrolytic capacitors.[1] They also have a much higher power density than batteries or fuel cells.
A typical D-cell-sized electrolytic capacitor may have capacitance of up to tens of millifarads. The same size EDLC might reach several farads, an improvement of two orders of magnitude. As of 2011[update] EDLCs had a maximum working voltage of a few volts (standard electrolytics can work at hundreds of volts) and capacities of up to 5,000 farads.[2] In 2010 the highest available EDLC specific energy was 30 Wh/kg (0.1 MJ/kg). [3] The amount of energy stored per unit of mass is called Specific energy, which is often measured in Watt-hour per kilogram (Wh/kg) or MegaJoules per kilogram (MJ/kg). Up to 85 Wh/kg has been achieved at room temperature in the lab[4], lower than rapid-charging lithium-titanate batteries.[5]
Much research is being carried out to improve performance; for example an order of magnitude energy density improvement was achieved in the laboratory in mid-2011.[6] Prices are dropping: a 3,000F capacitor that was US$5,000 ten years before was $50 in 2011.
EDLCs are used for energy storage rather than as general-purpose circuit components. They have a variety of commercial applications, notably in "energy smoothing" and momentary-load devices. They have applications as energy-storage and KERS devices used in vehicles, and for smaller applications like home solar energy systems where extremely fast charging is a valuable feature.
Contents
Concept
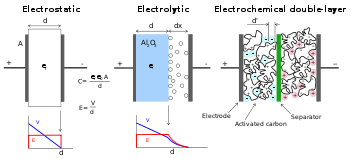 Comparison of construction diagrams of three capacitors. Left: "normal" capacitor, middle: electrolytic, right: electric double-layer capacitor
Comparison of construction diagrams of three capacitors. Left: "normal" capacitor, middle: electrolytic, right: electric double-layer capacitor
In a conventional capacitor, energy is stored by the removal of charge carriers, typically electrons, from one metal plate and depositing them on another. This charge separation creates a potential between the two plates, which can be harnessed in an external circuit. The total energy stored in this fashion increases with both the amount of charge stored and the potential between the plates. The amount of charge stored per unit voltage is essentially a function of the size, the distance, and the material properties of the plates and the material in between the plates (the dielectric), while the potential between the plates is limited by the breakdown field strength of the dielectric. The dielectric controls the capacitor's voltage. Optimizing the material leads to higher energy density for a given size of capacitor.
EDLCs do not have a conventional dielectric. Rather than two separate plates separated by an intervening insulator, these capacitors use virtual plates that are in fact two layers of the same substrate. Their electrochemical properties, the so-called "electrical double layer", result in the effective separation of charge despite the vanishingly thin (on the order of nanometers) physical separation of the layers. The lack of need for a bulky layer of dielectric, and the porosity of the material used, permits the packing of plates with much larger surface area into a given volume, resulting in high capacitances in practical-sized packages.
In an electrical double layer, each layer by itself is quite conductive, but the physics at the interface where the layers are effectively in contact means that no significant current can flow between the layers. However, the double layer can withstand only a low voltage, which means that electric double-layer capacitors rated for higher voltages must be made of matched series-connected individual EDLCs, much like series-connected cells in higher-voltage batteries.
EDLCs have much higher power density than batteries. Power density combines the energy density with the speed that the energy can be delivered to the load. Batteries, which are based on the movement of charge carriers in a liquid electrolyte, have [7] relatively slow charge and discharge times. Capacitors, on the other hand, can be charged or discharged at a rate that is typically limited by current heating of the electrodes.
So while existing EDLCs have energy densities that are perhaps 1/10 that of a conventional battery, their power density is generally 10 to 100 times as great. This makes them most suited to an intermediary role between electrochemical batteries and electrostatic capacitors, where neither sustained energy release nor immediate power demands dominate one another.
History
General Electric engineers experimenting with devices using porous carbon electrodes first observed the EDLC effect in 1957.[8] They believed that the energy was stored in the carbon pores and the device exhibited "exceptionally high capacitance", although the mechanism was unknown at that time.
General Electric did not immediately follow up on this work. In 1966 researchers at Standard Oil of Ohio developed the modern version of the devices, after they accidentally re-discovered the effect while working on experimental fuel cell designs.[9] Their cell design used two layers of activated charcoal separated by a thin porous insulator, and this basic mechanical design remains the basis of most electric double-layer capacitors.
Standard Oil did not commercialize their invention, licensing the technology to NEC, who finally marketed the results as “supercapacitors” in 1978, to provide backup power for maintaining computer memory.[9] The market expanded slowly for a time, but starting around the mid-1990s various advances in materials science and refinement of the existing systems led to rapidly improving performance and an equally rapid reduction in cost.
The first trials of supercapacitors in industrial applications were carried out for supporting the energy supply to robots.
In 2005 aerospace systems and controls company Diehl Luftfahrt Elektronik GmbH chose supercapacitors to power emergency actuation systems for doors and evacuation slides in airliners, including the new Airbus 380 jumbo jet.[10] In 2005, the ultracapacitor market was between US $272 million and $400 million, depending on the source.
As of 2007 all solid state micrometer-scale electric double-layer capacitors based on advanced superionic conductors had been for low-voltage electronics such as deep-sub-voltage nanoelectronics and related technologies (the 22 nm technological node of CMOS and beyond).[11]
Comparisons
Supercapacitors have several disadvantages and advantages relative to batteries, as described below.
Disadvantages
- The amount of energy stored per unit weight is generally lower than that of an electrochemical battery (3–5 W·h/kg for a standard ultracapacitor, although 85 W.h/kg has been achieved in the lab[4] as of 2010[update] compared to 30–40 W·h/kg for a lead acid battery), 100-250 W·h/kg for a lithium-ion battery and about 1/1,000th the volumetric energy density of gasoline.
- Has the highest dielectric absorption of any type of capacitor.
- High self-discharge – the rate is considerably higher than that of an electrochemical battery.
- Low maximum voltage – serial connections are needed to obtain higher voltages, and voltage balancing is required.
- Unlike practical batteries, the voltage across any capacitor, including EDLCs, drops significantly as it discharges. Effective storage and recovery of energy requires complex electronic control and switching equipment, with consequent energy loss. A detailed paper on a multi-voltage 5.3W EDLC power supply for medical equipment discusses design principles in detail. It uses a total of 55F of capacitance, charges in about 150 seconds, and runs for about 60 seconds. The circuit uses switch-mode voltage regulators followed by linear regulators for clean and stable power, reducing efficiency to about 70%. The authors discuss the types of switching regulator available, buck, boost, and buck-boost, and conclude that for the widely varying voltage across an EDLC buck-boost is best, boost second-best, and buck unsuitable[12].
- Very low internal resistance allows extremely rapid discharge when shorted, resulting in a spark hazard similar to any other capacitor of similar voltage and capacitance (generally much higher than electrochemical cells).
Advantages
- Long life, with little degradation over hundreds of thousands of charge cycles. Due to the capacitor's high number of charge-discharge cycles (millions or more compared to 200 to 1000 for most commercially available rechargeable batteries) it will last for the entire lifetime of most devices, which makes the device environmentally friendly. Rechargeable batteries wear out typically over a few years, and their highly reactive chemical electrolytes present a disposal and safety hazard. Battery lifetime can be optimised by charging only under favorable conditions, at an ideal rate and, for some chemistries, as infrequently as possible. EDLCs can help in conjunction with batteries by acting as a charge conditioner, storing energy from other sources for load balancing purposes and then using any excess energy to charge the batteries at a suitable time.
- Low cost per cycle
- Good reversibility
- Very high rates of charge and discharge.
- Extremely low internal resistance (ESR) and consequent high cycle efficiency (95% or more) and extremely low heating levels
- High output power
- High specific power. According to ITS (Institute of Transportation Studies, Davis, California) test results, the specific power of electric double-layer capacitors can exceed 6 kW/kg at 95% efficiency[13]
- Improved safety, no corrosive electrolyte and low toxicity of materials.
- Simple charge methods—no full-charge detection is needed; no danger of overcharging.
- When used in conjunction with rechargeable batteries, in some applications the EDLC can supply energy for a short time, reducing battery cycling duty and extending life
Materials
In general, EDLCs improve storage density through the use of a nanoporous material, typically activated charcoal, in place of the conventional insulating barrier. Activated charcoal is an extremely porous, "spongy" form of carbon with an extraordinarily high specific surface area — a common approximation is that 1 gram (a pencil-eraser-sized amount) has a surface area of roughly 250 m^2 — about the size of a tennis court. It is typically a powder made up of extremely fine but very "rough" particles, which, in bulk, form a low-density heap with many holes. As the surface area of even a thin layer of such a material is many times greater than a traditional material like aluminum, many more charge carriers (ions or radicals from the electrolyte) can be stored in a given volume. As carbon is not a good insulator (vs. the excellent insulators used in conventional devices), in general EDLCs are limited to low potentials on the order of 2–3 V, and thus must be "stacked" (connected in series), just as conventional battery cells must be, to supply higher voltages.
Activated charcoal is not the "perfect" material for this application. The charge carriers are actually (in effect) quite large—especially when surrounded by molecules—and are often larger than the holes left in the charcoal, which are too small to accept them, limiting the storage.
As of 2010[update] virtually all commercial supercapacitors use powdered activated carbon made from coconut shells.[citation needed] Higher performance devices are available, at a significant cost increase, based on synthetic carbon precursors that are activated with potassium hydroxide (KOH).
Research in EDLCs focuses on improved materials that offer higher usable surface areas.
- Graphene has excellent surface area per unit of gravimetric or volumetric densities, is highly conductive and can now be produced in various labs, but is not available in production quantities. Specific energy density of 85.6 Wh/kg at room temperature and 136 Wh/kg at 80 °C (all based on the total electrode weight), measured at a current density of 1 A/g have been observed. These energy density values are comparable to that of the Nickel metal hydride battery. The device makes full utilization of the highest intrinsic surface capacitance and specific surface area of single-layer graphene by preparing curved graphene sheets that do not restack face-to-face. The curved shape enables the formation of mesopores accessible to and wettable by environmentally benign ionic liquids capable of operating at a voltage >4 V.[14]
- Carbon nanotubes have excellent nanoporosity properties, allowing tiny spaces for the polymer to sit in the tube and act as a dielectric.[15] Carbon nanotubes can store about the same charge as charcoal (which is almost pure carbon) per unit surface area but nanotubes can be arranged in a more regular pattern that exposes greater suitable surface area.[16] The addition of carbon nanotubes in capacitors can greatly improve and enhance the performance of electric double-layer capacitors. Due to the high surface area and high conductivity of single-wall carbon nanotubes, the addition of these nanotubes allows optimization for these capacitors.[17] Multi-walled carbon nanotubes have a presence of mesopores that allow for easy access of ions at the electrode/electrolyte interface. The thin walls of a carbon nanotube allow for high capacitance in an electric double-layer capacitor.[18] By adding multi-walled nanotubes to these capacitors, the resistance of the electrodes can be decreased. The capacitor cells with multi-walled nanotube fibers had higher electron and electrolyte-ion conductivities as compared to cells that did not have these nanotubes. These nanotubes also improved the power capabilities of the capacitors.[19]
- Some polymers (e.g. polyacenes and conducting polymers) have a redox (reduction-oxidation) storage mechanism along with a high surface area.
- Carbon aerogel provides extremely high surface area gravimetric densities of about 400–1000 m²/g. The electrodes of aerogel supercapacitors are a composite material usually made of non-woven paper made from carbon fibers and coated with organic aerogel, which then undergoes pyrolysis. The carbon fibers provide structural integrity and the aerogel provides the required large surface area. Small aerogel supercapacitors are being used as backup electricity storage in microelectronics. Aerogel capacitors can only work at a few volts; higher voltages ionize the carbon and damage the capacitor. Carbon aerogel capacitors have achieved 325 J/g (90 W·h/kg) energy density and 20 W/g power density.[20]
- Solid activated carbon, also termed consolidated amorphous carbon (CAC). It can have a surface area exceeding 2800 m2/g and may be cheaper to produce than aerogel carbon.[21]
- Tunable nanoporous carbon exhibits systematic pore size control. H2 adsorption treatment can be used to increase the energy density by as much as 75% over what was commercially available as of 2005[update].[22][23]
- Mineral-based carbon is a nonactivated carbon, synthesised from metal or metalloid carbides, e.g. SiC, TiC, Al4C3.[24] The synthesised nanostructured porous carbon, often called Carbide Derived Carbon (CDC), has a surface area of about 400 m²/g to 2000 m²/g with a specific capacitance of up to 100 F/mL (in organic electrolyte). As of 2006[update] this material was used in a supercapacitor with a volume of 135 mL and 200 g weight having 1.6 kF capacitance. The energy density is more than 47 kJ/L at 2.85 V and power density of over 20 W/g.[25]
- In August 2007 researchers combined a biodegradable paper battery with aligned carbon nanotubes, designed to function as both a lithium-ion battery and a supercapacitor (called bacitor). The device employed an ionic liquid, essentially a liquid salt, as the electrolyte. The paper sheets can be rolled, twisted, folded, or cut with no loss of integrity or efficiency, or stacked, like ordinary paper (or a voltaic pile), to boost total output. They can be made in a variety of sizes, from postage stamp to broadsheet. Their light weight and low cost make them attractive for portable electronics, aircraft, automobiles, and toys (such as model aircraft), while their ability to use electrolytes in blood make them potentially useful for medical devices such as pacemakers.[26]
- Other teams are experimenting with custom materials made of activated polypyrrole, and nanotube-impregnated papers.
Properties
The properties of EDLCs are being improved as new research progresses.
Capacitance
The capacitance of EDLCs was up to several thousands of farads as of 2011[update].
Voltage
As of 2011[update] EDLCs rated up to a maximum working voltage of not more than about 5V were available.
Specific energy
The specific energy of existing commercial EDLCs ranges from around 0.5 to 30 W·h/kg[27][28] including lithium ion capacitors, known also as a "hybrid capacitor". Experimental electric double-layer capacitors have demonstrated specific energies of 30 W·h/kg and have been shown to be scalable to at least 136 W·h/kg.[29][30] For comparison, a conventional lead-acid battery stores typically 30 to 40 W·h/kg and modern lithium-ion batteries about 160 W·h/kg. Gasoline has a net calorific value (NCV) of around 12,000 W·h/kg; automobile applications operate at about 20% tank-to-wheel efficiency, giving an effective specific energy of 2,400 W·h/kg (effective specific energy of ultracapacitors is not known since data is not available for electrical-output-to-wheel efficiency for ultracapacitor driven vehicles).
Power density
EDLCs have energy densities perhaps one tenth that of a rechargeable battery, but power densities typically 10 to 100 times greater.
Self-discharge
An EDLC which is charged and stored loses its charge (self-discharge) much faster than a typical electrolytic capacitor, and somewhat faster than a rechargeable battery.
Price
Research and development bring rapid improvements in price as well as physical properties.
Costs have fallen quickly, with cost per kilojoule dropping faster than cost per farad. By 2006 the cost of supercapacitors was 1 cent per farad and $2.85 per kilojoule and dropping.[31] A 3,000F capacitor that was US$5,000 ten years before was $50 in 2011.[32]
Applications
Vehicles
Heavy and public transport
See also: Capa vehicleSome of the earliest uses were motor startup capacitors for large engines in tanks and submarines, and as the cost has fallen they have started to appear on diesel trucks and railroad locomotives.[33][34] In the 2000s they attracted attention in the green energy world, where their ability to charge much faster than batteries makes them particularly suitable for regenerative braking applications. New technology in development[update] could potentially make EDLCs with high enough energy density to be an attractive replacement for batteries in all-electric cars and plug-in hybrids, as EDLCs charge quickly and are stable with respect to temperature.
China is experimenting with a new form of electric bus (capabus) that runs without powerlines using large onboard EDLCs, which quickly recharge whenever the bus is at any bus stop (under so-called electric umbrellas), and fully charge in the terminus. A few prototypes were being tested in Shanghai in early 2005. In 2006, two commercial bus routes began to use electric double-layer capacitor buses; one of them is route 11 in Shanghai.[35]
In 2001 and 2002 VAG, the public transport operator in Nuremberg, Germany tested an hybrid bus that uses a diesel-electric battery drive system with electric double-layer capacitors.[36] Since 2003 Mannheim Stadtbahn in Mannheim, Germany has operated a light-rail vehicle (LRV) that uses EDLCs to store braking energy.[37][38]
Other public transport manufacturers are developing EDLC technology, including mobile storage[39] and a stationary trackside power supply.[40][41]
A triple hybrid forklift truck uses fuel cells and batteries as primary energy storage and EDLCs to supplement this energy storage solution.[42]
Automotive
Ultracapacitors are used in some concept prototype vehicles, in order to keep batteries within resistive heating limits and extend battery life.[43][44] The ultrabattery combines a supercapacitor and a battery in one unit, creating an electric vehicle battery that lasts longer, costs less and is more powerful than current plug-in hybrid electric vehicles (PHEVs).[45][46]
Motor racing
The FIA, the governing body for many motor racing events, proposed in the Power-Train Regulation Framework for Formula 1 version 1.3 of 23 May 2007 that a new set of power train regulations be issued that includes a hybrid drive of up to 200 kW input and output power using "superbatteries" made with both batteries and supercapacitors.[47]
Complementing batteries
When used in conjunction with rechargeable batteries in uninterruptible power supplies and similar applications, the EDLC can handle short interruptions, requiring the batteries to be used only during long interruptions, reducing the cycling duty and extending their life[48]
Low-power applications
EDLCs can be used to operate low-power equipment such as PC Cards, photographic flash, flashlights, portable media players, and automated meter reading equipment.[49] They are advantageous when extremely fast charging is required. In professional medical applications, EDLCs have been used to power a handheld, laser-based breast cancer detector (55F to provide 5.3W at multiple voltages; charges in 150", runs for 60").[12]
In 2007 a cordless electric screwdriver that uses an EDLC for energy storage was produced.[50] It charges in 90 seconds, retains 85% of the charge after 3 months, and holds enough charge for about half the screws (22) a comparable screwdriver with a rechargeable battery will handle (37). Two LED flashlights using EDLCs were released in 2009. They charge in 90 seconds.[51]
Alternative energy
The idea of replacing batteries with capacitors in conjunction with novel energy sources became a conceptual umbrella of the Green Electricity (GEL) Initiative.[52] One successful GEL Initiative concept was a muscle-driven autonomous solution that employs a multi-farad EDLC as energy storage to power a variety of portable electrical and electronic devices such as MP3 players, AM/FM radios, flashlights, cell phones, and emergency kits.[53]
Market
According to Innovative Research and Products (iRAP), ultracapacitor market growth will continue during 2009 to 2014. Worldwide business, over US$275 million in 2009, will continue to grow at an AAGR of 21.4% through 2014.[54]
See also
- Electric vehicle battery
- Types of capacitors
- Nanoflower
- Rechargeable electricity storage system
- Flywheel energy storage
- List of emerging technologies
- Lithium ion capacitor
- Self-powered equipment
- Mechanically powered flashlight
References
- ^ Garthwaite, Josie (12 July 2011). "How ultracapacitors work (and why they fall short)". GigaOM's Earth2Tech. http://gigaom.com/cleantech/how-ultracapacitors-work-and-why-they-fall-short/. Retrieved 13 July 2011.
- ^ 5000F, Nesscap Products
- ^ A 30 Wh/kg Supercapacitor for Solar Energy and a New Battery. Jeol.com (3 October 2007). Retrieved on 13 September 2011.
- ^ a b Graphene supercapacitor breaks storage record. physicsworld.com. Retrieved on 13 September 2011.
- ^ Note: all references to batteries in this article should be taken to refer to rechargeable, not primary (aka disposable), batteries.
- ^ Chemistry World: New carbon material boosts supercapacitors. Rsc.org. 13 May 2011. Retrieved on 13 September 2011.
- ^ Garthwaite, Josie (12 July). "How ultracapacitors work (and why they fall short)". Earth2Tech. GigaOM Network. http://gigaom.com/cleantech/how-ultracapacitors-work-and-why-they-fall-short/. Retrieved 13 July 2011.
- ^ US 2800616, Becker, H.I., "Low voltage electrolytic capacitor", issued 1957-07-23
- ^ a b The Charge of the Ultra – Capacitors. IEEE Spectrum, November 2007
- ^ Boostcap (of Maxwell Technologies)
- ^ Высокоёмкие конденсаторы для 0,5 вольтовой наноэлектроники будущего. Nanometer.ru. 17 October 2007. Retrieved on 13 September 2011.
- ^ a b TurboCap: A Batteryless, Supercapacitor-based Power Supply, Chulsung Park, Keunsik No, and Pai H. Chou, University of California, USA and National Tsing Hua University, Taiwan. Retrieved on 13 September 2011.
- ^ Prototype Test APowerCap press release: Results highly appreciated by Ultracapacitor Experts, 2006.
- ^ Liu, Chenguang; Yu, Zhenning; Jang, Bor Z.; Zhamu, Aruna; Jang, Bor Z. (2010). "Graphene-Based Supercapacitor with an Ultrahigh Energy Density". Nano Letters (American Chemical Society) 10 (12): 4863–4868. doi:10.1021/nl102661q.
- ^ MIT LEES on Batteries. MIT press release, 2006.
- ^ Researchers fired up over new battery, Deborah Halber, MIT News Office, 8 February 2006
- ^ Arepalli, S.; H. Fireman, C. Huffman, P. Moloney, P. Nikolaev, L. Yowell, C.D. Higgins, K. Kim, P.A. Kohl, S.P. Turano, and W.J. Ready (2005). "Carbon-Nanotube-Based Electrochemical Double-Layer Capacitor Technologies for Spaceflight Applications". JOM: 24–31.
- ^ Du, C. S.; Pan N. J. Power Sources 2006, 160, 1487–1494
- ^ Dillon, A.C. (2010). "Carbon Nanotubes for Photoconversion and Electrical Energy Storage". Chem. Rev. 110 (11): 6856–6872. doi:10.1021/cr9003314. PMID 20839769.
- ^ Lerner EJ, "Less is more with aerogels: A laboratory curiosity develops practical uses". The Industrial Physicist (2004)
- ^ Reticle US 6787235
- ^ Yushin, G., Dash, R.K., Jagiello, J., Fischer, J.E., & Gogotsi, Y. (2006). Carbide derived carbons: effect of pore size on hydrogen storage and heat of adsorption. Advanced Functional Materials, 16(17), 2288–2293, Retrieved from http://nano.materials.drexel.edu/Papers/200500830.pdf
- ^ Y-Carbon. Y-carbon.us. Retrieved on 13 September 2011.
- ^ US 6602742 and WO 2005118471
- ^ Latest developments in carbide derived carbon (2006)
- ^ Beyond batteries: storing power in a sheet of paper. Rensselaer Polytechnic Institute press release (13 August 2007)
- ^ Advanced Capacitor Technologies, Inc. ( ACT ). Act.jp. Retrieved on 13 September 2011.
- ^ Ultracapacitor – Google Video – une vidéo Techniek en wetenschap. Dailymotion. Retrieved on 13 September 2011.
- ^ Liu, Chenguang; Yu, Zhenning; Neff, David; Zhamu, Aruna; Jang, Bor Z. (2010). "Graphene-Based Supercapacitor with an Ultrahigh Energy Density". Nano Letters 10 (12): 4863. doi:10.1021/nl102661q.
- ^ Carbon Nanotube Enhanced Ultracapacitors, MIT LEES ultracapacitor project
- ^ Supercapacitors see growth as costs fall. Electronics Weekly. 3 March 2006. Retrieved on 13 September 2011.
- ^ Advent of Ultracapacitors Signals Change in Wind Turbine Capabilities. renewableenergyworld.com. March 2011. Retrieved on 13 September 2011.
- ^ Supercapacitors, US DoE overview[dead link]
- ^ "Ultracapacitors". Office of Energy Efficiency and Renewable Energy. 15 April 2009. http://www1.eere.energy.gov/vehiclesandfuels/technologies/energy_storage/ultracapacitors.html. Retrieved 10 January 2011. "Using an ultracapacitor in conjunction with a battery combines the power performance of the former with the greater energy storage capability of the latter. It can extend the life of a battery, save on replacement and maintenance costs, and enable a battery to be downsized."
- ^ [1] (in Chinese, archived page)
- ^ VAG Verkehrs-AG Nürnberg. En.vag.de. Retrieved on 13 September 2011.
- ^ UltraCaps win out in energy storage. Richard Hope, Railway Gazette International July 2006
- ^ M. Steiner. MITRAC Energy Saver. Bombardier presentation (2006).
- ^ The Transportation Systems division of Siemens AG is developing mobile energy storage called Sibac Energy Storage Siemens AG Sibac ES[dead link] Sibac ES Product Page (as of November 2007)
- ^ Sitras SES Sitras SES Sitras SES Product Page (as of November 2007)
- ^ Cegelec a.s. | Electrical equipment for municipal mass transit | utilization of regenerated energy | transport. Cegelec.cz. Retrieved on 13 September 2011.
- ^ Proton Power Systems Unveils the World’s First Triple-hybrid Forklift Truck. Fuel Cell Works press release (2007).
- ^ AFS Trinity Wald, Matthew L. (13 January 2008). "Closing the Power Gap Between a Hybrid's Supply and Demand". The New York Times. http://www.nytimes.com/2008/01/13/automobiles/13ULTRA.html. Retrieved 1 May 2010.
- ^ AFS TRINITY UNVEILS 150 MPG EXTREME HYBRID (XH™) SUV. (PDF) . AFS Trinity Power Corporation. 13 January 2008. Retrieved on 13 September 2011.
- ^ . http://www.csiro.au/science/Ultra-Battery.html.
- ^ "Ultracapacitors". Office of Energy Efficiency and Renewable Energy. 15 April 2009. http://www1.eere.energy.gov/vehiclesandfuels/technologies/energy_storage/ultracapacitors.html. Retrieved 10 January 2011. "Many applications can benefit from ultracapacitors, especially HEVs and PHEVs. Ultracapacitors can be the primary energy source during acceleration and hill climbing, as well as for recovery of braking energy because they are excellent at providing quick bursts of energy."
- ^ Formula One 2011: Power-Train Regulation Framework. (PDF) . 24 May 2007. Retrieved on 13 September 2011.
- ^ Using SuperCapacitors for Energy Storage, 2010. discoversolarenergy.com. Retrieved on 13 September 2011.
- ^ Graham Pitcher If the cap fits ... New Electronics. 26 March 2006
- ^ Coleman FlashCell Cordless Screwdriver. Ohgizmo.com (1 October 2007). Retrieved on 13 September 2011.
- ^ Ultracapacitor LED Flashlight Charges In 90 Seconds
- ^ Alexander Bell Green Electricity (GEL) Initiative,Mobile Electricity: Battery-free, renewable, clean (revised as of 11 July 2006)
- ^ Muscle power drives battery-free electronics (Alexander Bell, EDN, 21 November 2005)
- ^ Ultracapacitors For Stationary, Industrial, Consumer And Transport Energy Storage – An Industry, Technology And Market Analysis
External links
- Super Capacitor Seminar
- Article on ultracapacitors at electronicdesign.com
- Article on ultracapacitors at batteryuniversity.com
- A new version of an old idea is threatening the battery industry (The Economist).
- An Encyclopedia Article From the Yeager center at CWRU.
- Ultracapacitors & Supercapacitors Forum
- Special Issue of Interface magazine on electrochemical capacitors
- Nanoflowers Improve Ultracapacitors: A novel design could boost energy storage (Technology Review) and Can nanoscopic meadows drive electric cars forward? (New Scientist)
- If the cap fits... How supercapacitors can help to solve power problems in portable products.
- A web that describes the development of solid-state and hybrid supercapacitors from CNR-ITAE (Messina) Italy
Categories:- Capacitors
- Emerging technologies
- Energy conversion
Wikimedia Foundation. 2010.


