- Osmotic power
-
Renewable energy Biofuel
Biomass
Geothermal
Hydroelectricity
Solar energy
Tidal power
Wave power
Wind powerOsmotic power or salinity gradient power is the energy available from the difference in the salt concentration between seawater and river water. Two practical methods for this are reverse electrodialysis[1] (RED) and pressure-retarded osmosis.[2] (PRO).
Both processes rely on osmosis with ion specific membranes. The key waste product is brackish water. This byproduct is the result of natural forces that are being harnessed: the flow of fresh water into seas that are made up of salt water.
The technologies have been confirmed in laboratory conditions. They are being developed into commercial use in the Netherlands (RED) and Norway (PRO). The cost of the membrane has been an obstacle. A new, cheap membrane, based on an electrically modified polyethylene plastic, made it fit for potential commercial use.[3]
Other methods have been proposed and are currently under development. Among them, a method based on electric double-layer capacitor technology.[4] and a method based on vapor pressure difference.[5]
The world's first osmotic power plant with capacity of 4 kW was opened by Statkraft on 24 November 2009 in Tofte, Norway.[6][7][8] This plant uses polyimide as a membrane, and is able to produce 1W/m² of membrane. This amount of power is obtained at 10 l of water flowing through the membrane per second, and at a pressure of 10 bar. Both the increasing of the pressure as well as the flow rate of the water would make it possible to increase the power output. Hypothetically, the output of the SGP-plant could easily be doubled.[9][10]
Contents
Basics of salinity gradient power
Salinity gradient power is a specific renewable energy alternative that creates renewable and sustainable power by using naturally occurring processes. This practice does not contaminate or release carbon dioxide (CO2) emissions (vapor pressure methods will release dissolved air containing CO2 at low pressures—these non-condensable gases can be re-dissolved of course, but with an energy penalty). Also as stated by Jones and Finley within their article “Recent Development in Salinity Gradient Power”, there is basically no fuel cost.
Salinity gradient energy is based on using the resources of “osmotic pressure difference between fresh water and sea water.”[11] All energy that is proposed to use salinity gradient technology relies on the evaporation to separate water from salt. Osmotic pressure is the "chemical potential of concentrated and dilute solutions of salt".[12] When looking at relations between high osmotic pressure and low, solutions with higher concentrations of salt have higher pressure.
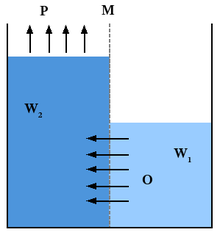
Differing salinity gradient power generations exist but one of the most commonly discussed is Pressure Retarded Osmosis (PRO).This method of generating power was invented by Prof. Sidney Loeb in 1973 at the Ben-Gurion University of the Negev, Beersheba, Israel.[13] Within PRO seawater is pumped into a pressure chamber where the pressure is lower than the difference between fresh and salt water pressure. Fresh water moves in a semipermeable membrane and increases its volume in the chamber. As the pressure in the chamber is compensated a turbine spins to generate electricity. In Braun's article he states that this process is easy to understand in a more broken down manner. Two solutions, A being salt water and B being fresh water are separated by a membrane. He states "only water molecules can pass the semipermeable membrane. As a result of the osmotic pressure difference between both solutions, the water from solution B thus will diffuse through the membrane in order to dilute the solution".[14] The pressure drives the turbines and power the generator that produces the electrical energy.
Osmosis might be used directly to "pump" fresh water out of The Netherlands into the sea. This is currently done using electric pumps.
Methods
While the mechanics and concepts of salinity gradient power are still being studied, the power source has been implemented in several different locations. Most of these are experimental, but thus far they have been predominantly successful. The various companies that have utilized this power have also done so in many different ways as there are several concepts and processes that harness the power from salinity gradient.
Pressure-retarded osmosis
One method to utilize salinity gradient energy is called pressure-retarded osmosis.[15] In this method, seawater is pumped into a pressure chamber that is at a pressure lower than the difference between the pressures of saline water and fresh water. Freshwater is also pumped into the pressure chamber through a membrane, which increase both the volume and pressure of the chamber. As the pressure differences are compensated, a turbine is spun creating energy. This method is being specifically studied by the Norwegian utility Statkraft, which has calculated that up to 25 TWh/yr would be available from this process in Norway.[16] Statkraft has built the world's first prototype osmotic power plant on the Oslo fiord which was opened by Her Royal Highness Crown Princess Mette-Marit of Norway[17] on November 24, 2009. It aims to produce enough electricity to light and heat a small town within five years by osmosis. At first it will produce a minuscule 4 kilowatts – enough to heat a large electric kettle, but by 2015 the target is 25 megawatts – the same as a small wind farm.[18]
Reversed electrodialysis
A second method being developed and studied is reversed electrodialysis or reverse dialysis, which is essentially the creation of a salt battery. This method was described by Weinstein and Leitz as “an array of alternating anion and cation exchange membranes can be used to generate electric power from the free energy of river and sea water.”
The technology related to this type of power is still in its infant stages, even though the principle was discovered in the 1950s. Standards and a complete understanding of all the ways salinity gradients can be utilized are important goals to strive for in order make this clean energy source more viable in the future.
Capacitive method
A third method is Doriano Brogioli's[4] capacitive method, which is relatively new and has so far only been tested on lab scale. With this method energy can be extracted out of the mixing of saline water and freshwater by cyclically charging up electrodes in contact with saline water, followed by a discharge in freshwater. Since the amount of electrical energy which is needed during the charging step is less than one gets out during the discharge step, each completed cycle effectively produces energy. An intuitive explanation of this effect is that the great number of ions in the saline water efficiently neutralizes the charge on each electrode by forming a thin layer of opposite charge very close to the electrode surface, known as an electric double layer. Therefore, the voltage over the electrodes remains low during the charge step and charging is relatively easy. In between the charge and discharge step, the electrodes are brought in contact with freshwater. After this, there are less ions available to neutralize the charge on each electrode such that the voltage over the electrodes increases. The discharge step which follows is therefore able to deliver a relatively high amount of energy. A physical explanation is that on an electrically charged capacitor, there is a mutually attractive electric force between the electric charge on the electrode, and the ionic charge in the liquid. In order to pull ions away from the charged electrode, osmotic pressure must do work. This work done increases the electrical potential energy in the capacitor. An electronic explanation is that capacitance is a function of ion density. By introducing a salinity gradient and allowing some of the ions to diffuse out of the capacitor, this reduces the capacitance, and so the voltage must increase, since the voltage equals the ratio of charge to capacitance.
Absorption refrigeration cycle
For the purpose of dehumidifying air, in a water-spray absorption refrigeration system, water vapor is dissolved into a deliquescent salt water mixture using osmotic power as an intermediary. The primary power source originates from a thermal difference, as part of a thermodynamic heat engine cycle.
Solar pond
At the Eddy Potash Mine in New Mexico, a technology called "salinity gradient solar pond" (SGSP) is being utilized to provide the energy needed by the mine. This method does not harness osmotic power, only solar power (see: solar pond). Sunlight reaching the bottom of the saltwater pond is absorbed as heat. The effect of natural convection, wherein "heat rises", is blocked using density differences between the three layers that make up the pond, in order to trap heat. The upper convection zone is the uppermost zone, followed by the stable gradient zone, then the bottom thermal zone. The stable gradient zone is the most important. The saltwater in this layer can not rise to the higher zone because the saltwater above has lower salinity and is therefore less-dense and more buoyant; and it can not sink to the lower level because that saltwater is denser. This middle zone, the stable gradient zone, effectively becomes an "insulator" for the bottom layer (although the main purpose is to block natural convection, since water is a poor insulator). This water from the lower layer, the storage zone, is pumped out and the heat is used to produce energy, usually by turbine in an organic Rankine cycle.[19]
In theory a solar pond could be used to generate osmotic power if evaporation from solar heat is used to create a salinity gradient, and the potential energy in this salinity gradient is harnessed directly using one of the first three methods above, such as the capacitive method.
Possible negative environmental impact
Marine and river environments have obvious differences in water quality, namely salinity. Each species of aquatic plant and animal is adapted to survive in either marine, brackish, or freshwater environments. There are species that can tolerate both, but these species usually thrive best in a specific water environment. The main waste product of salinity gradient technology is brackish water. The discharge of brackish water into the surrounding waters, if done in large quantities and with any regularity, will cause salinity fluctuations. While some variation in salinity is usual, particularly where fresh water (rivers) empties into an ocean or sea anyway, these variations become less important for both bodies of water with the addition of brackish waste waters. Extreme salinity changes in an aquatic environment may result in findings of low densities of both animals and plants due to intolerance of sudden severe salinity drops or spikes.[20] According to the prevailing environmentalist opinions, the possibility of these negative effects should be considered by the operators of future large blue energy establishments.
Furthermore, impingement and entrainment at intake structures are a concern due to large volumes of both river and sea water utilized in both PRO and RED schemes. Intake construction permits must meet strict environmental regulations and desalination plants and power plants that utilize surface water are sometimes involved with various local, state and federal agencies to obtain permission that can take upwards to 18 months.
Finally, some scientists have predicted that if China does not check their irrigation withdrawals from rivers, ALL Chinese rivers will not meet the ocean at least during some part of the year by 2025. This has already happened with the mother of Chinese rivers, the Yellow river. An investment in osmotic power must consider future upstream use in the long-run.
See also
- Forward osmosis
- Electrodialysis reversal (EDR)
- Reversed electrodialysis
- Reverse osmosis
- Semipermeable membrane
- Green energy
- Renewable energy
- Fugacity
- Concentration cell
- Solar Pond
References
- ^ Power generation by reverse electrodialysis
- ^ How does PRO work?
- ^ History of osmotic power (PDF) at archive.org
- ^ a b D. Brogioli, Extracting renewable energy from a salinity difference using a capacitor, Phys. Rev. Lett. 103 058501-1-4 (2009).
- ^ M. Olsson, G. L. Wick and J. D. Isaacs, Salinity Gradient Power: utilizing vapour pressure differences, Science 206 452--454 (1979)
- ^ John Gartner (2009-11-24). "World's First Osmotic Power Plant Opens". Reuters. http://www.reuters.com/article/2009/11/24/us-norway-osmotic-idUSTRE5AN20Q20091124. Retrieved 2011-04-25.
- ^ November 24, 2009, cnet.com: Norway opens world's first osmotic power plant
- ^ 30 November 2009, itnsource.com: NORWAY: World's first osmotic power plant opens in Tofte
- ^ US lab reaching 2,7W/m² in PRO SGP-plant
- ^ Natuurwetenshap & Techniek magazine, February 2010
- ^ (Jones, A.T., W. Finley. “Recent developments in salinity gradient power”. Oceans. 2003. 2284-2287.)
- ^ (Brauns, E. “Toward a worldwide sustainable and simultaneous large-scale production of renewable energy and potable water trough salinity gradient power by combining reversed electrodialysis and solar power?” Environmental Process and Technology. Jan 2007. 312-323.)
- ^ Israel Patent Application 42658 of July 3, 1973. (see also US patent 3,906,250 granted September 16, 1975. Erroneously shows Israel priority as 1974 instead of 1973).
- ^ (Brauns, E. “Toward a worldwide sustainable and simultaneous large-scale production of renewable energy and potable water through salinity gradient power by combining reversed electrodialysis and solar power?.” Environmental Process and Technology. Jan 2007. 312-323.)
- ^ Salinity-gradient power: Evaluation of pressure-retarded osmosis and reverse electrodialysis
- ^ Recent Developments in Salinity Gradient Power
- ^ [1] Statkraft-osmotic-power
- ^ http://news.bbc.co.uk/1/hi/world/europe/8377186.stm BBC News Norway's Statkraft opens first osmotic power plant
- ^ Salinity Gradient Solar Pond Technology Applied to Potash Solution Mining
- ^ Montague, C., Ley, J. A Possible Effect of Salinity Fluctuation on Abundance of Benthic Vegetation and Associated Fauna in Northeastern Florida Bay. Estuaries and Coasts. 1993. Springer New York. Vol.15 No. 4. Pg. 703-717
External links
Ocean energy Wave power Australia • New Zealand
Tidal power Osmotic power Other Marine current power • Offshore construction • Ocean thermal energy conversion • Pelamis wave energy converter • • SDE Sea Waves Power Plant • Wind power (offshore) • Wave farmPortals: Energy • Renewable energy • Sustainable development Fuel cells Types Alkaline fuel cell · Blue energy · Direct borohydride fuel cell · Direct carbon fuel cell · Direct-ethanol fuel cell · Direct methanol fuel cell · Electro-galvanic fuel cell · Enzymatic biofuel cell · Formic acid fuel cell · Flow battery · Molten carbonate fuel cell · Microbial fuel cell · Metal hydride fuel cell · Phosphoric acid fuel cell · Protonic ceramic fuel cell · Photoelectrochemical cell · Proton exchange membrane fuel cell · Regenerative fuel cell · Reformed methanol fuel cell · Solid oxide electrolyser cell · Solid oxide fuel cell · Unitized regenerative fuel cell · Zinc-air batteryHydrogen Categories:- Fuel cells
- Sustainable technologies
- Alternative energy
- Energy conversion
- Water power
- Membrane technology
Wikimedia Foundation. 2010.