- Seawater
-
 Seawater in the Strait of Malacca
Seawater in the Strait of Malacca
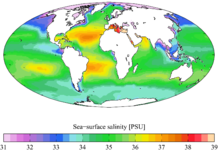
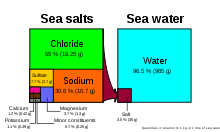
Seawater is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, or 599 mM). This means that every kilogram (roughly one litre by volume) of seawater has approximately 35 grams (1.2 oz) of dissolved salts (predominantly sodium (Na+
) and chloride (Cl−
) ions). The average density of seawater at the ocean surface is 1.025 g/ml. Seawater is denser than both fresh water and pure water (density 1.0 g/ml @ 4 °C (39 °F)) because the dissolved salts add mass without contributing significantly to the volume. The freezing point of seawater decreases as salt concentration increases. At a typical salinity it freezes at about −2 °C (28 °F).[1] The coldest seawater ever recorded (in a liquid state) was in 2010, in a stream under an Antarctic glacier, and measured −2.6 °C (27.3 °F).[2]Contents
Salinity
Main article: SalinitySeawater composition (by mass) (salinity = 35) Element Percent Element Percent Oxygen 85.84 Sulfur 0.091 Hydrogen 10.82 Calcium 0.04 Chloride 1.94 Potassium 0.04 Sodium 1.08 Bromine 0.0067 Magnesium 0.1292 Carbon 0.0028 Although the vast majority of seawater has a salinity of between 3.1% and 3.8%, seawater is not uniformly saline throughout the world. Where mixing occurs with fresh water runoff from river mouths or near melting glaciers, seawater can be substantially less saline. The most saline open sea is the Red Sea, where high rates of evaporation, low precipitation and river inflow, and confined circulation result in unusually salty water. The salinity in isolated bodies of water (for example, the Dead Sea) can be considerably greater still.
The density of surface seawater ranges from about 1,020 to 1,029 kg•m−3, depending on the temperature and salinity. Deep in the ocean, under high pressure, seawater can reach a density of 1,050 kg•m−3 or higher. Seawater pH is limited to the range 7.5 to 8.4. The speed of sound in seawater is about 1,500 metres/second, and varies with water temperature, salinity, and pressure.
Compositional differences from fresh water
Seawater contains more dissolved ions than all types of freshwater.[4] However, the ratios of various solutes differ dramatically. For instance; although seawater contains about 2.8 times the bicarbonate than river water based on molarity, the percentage of bicarbonate in seawater as a ratio of all dissolved ions is far lower than in river water. Bicarbonate ions also constitute 48% of river water solutes, but only 0.14% of all seawater ions.[4][5] Differences like these are due to the varying residence times of seawater solutes; sodium and chlorine have very long residence times, while calcium (vital for carbonate formation) tends to precipitate much more quickly.[5] The most abundant dissolved ions in seawater are sodium, chloride, magnesium, sulfate and calcium.[6]
Geochemical explanations
Total Molar Composition of Seawater (Salinity = 35)[7] Component Concentration (mol/kg) H2O 53.6 Cl− 0.546 Na+ 0.469 Mg2+ 0.0528 SO2−
40.0282 Ca2+ 0.0103 K+ 0.0102 CT 0.00206 Br− 0.000844 BT 0.000416 Sr2+ 0.000091 F− 0.000068 Scientific theories behind the origins of sea salt started with Sir Edmond Halley in 1715, who proposed that salt and other minerals were carried into the sea by rivers after rainfall washed it out of the ground. Upon reaching the ocean, these salts concentrated as the process of evaporation (see Hydrologic cycle) removed the water. Halley noted that most lakes that don’t have ocean outlets (such as the Dead Sea and the Caspian Sea, see endorheic basin), have high salt content. Halley termed this process "continental weathering".
Halley's theory is partly correct. In addition, sodium leached out of the ocean floor when the ocean formed. The presence of salt’s other dominant ion, chloride, results from outgassing of chloride (as hydrochloric acid) with other gases from Earth's interior via volcanos and hydrothermal vents. The sodium and chloride ions subsequently became the most abundant constituents of sea salt.
Ocean salinity has been stable for billions of years, most likely as a consequence of a chemical/tectonic system which removes as much salt as is deposited; for instance, sodium and chloride sinks include evaporite deposits, pore water burial, and reactions with seafloor basalts.[8] Since the ocean's formation, sodium no longer leaches from the ocean floor, but instead is captured in sedimentary layers covering the ocean bed. One theory is that plate tectonics forces salt under the continental land masses, where it slowly leaches again to the surface.
Human consumption of seawater
Accidentally consuming small quantities of clean seawater is not harmful, especially if the seawater is consumed along with a larger quantity of fresh water. However, drinking seawater to maintain hydration is counterproductive; more water must be excreted to eliminate the salt (via urine) than the amount of water that is gained from drinking the seawater itself.[9]
This occurs because the renal system actively regulates human blood’s sodium chloride within a very narrow range around 9 g/L (0.9% by weight). Seawater contains varying concentrations of dissolved sodium chloride, depending on its source, ranging from about 2% in parts of the Baltic, to over 4% in parts of the eastern Mediterranean and Red sea. In most open waters concentrations vary somewhat around typical values of about 3.5%, all of them far higher than the body can tolerate in the blood, and most of them beyond what the kidney can deal with. A point frequently overlooked in optimistic arguments that the kidney can in fact excrete NaCl in Baltic concentrations, is that the gut cannot absorb water at such concentrations, so that logically there should be no profit in drinking seawater. At best, drinking seawater temporarily increases blood’s concentration of sodium chloride. This in turn signals the kidney to excrete sodium, but seawater’s sodium concentration is above the kidney’s maximum concentrating ability. Eventually the blood’s sodium concentration will rise to toxic levels, removing water from all cells and interfering with nerve conduction, ultimately producing fatal seizure and heart arrhythmia.
Survival manuals consistently advise against drinking seawater. For example, the book "Medical Aspects of Harsh Environments" (Chapter 29 - Shipboard Medicine)[10] presents a summary of 163 life raft voyages. The risk of death was 39% for those who drank seawater, compared to only 3% for those who did not drink seawater. The effect of seawater intake has also been studied in laboratory settings in rats.[11] This study confirmed the negative effects of drinking seawater when dehydrated.
The temptation to drink seawater has always been greatest for sailors who have expended their supply of fresh water, and are unable to capture enough rainwater for drinking. This frustration is described famously by a line from Samuel Taylor Coleridge's The Rime of the Ancient Mariner:
- "Water, water, everywhere,
- And all the boards did shrink;
- Water, water, everywhere,
- Nor any drop to drink."
Although it is clear that a human cannot survive on seawater alone, some people claim that one can drink up to two cups a day, mixed with fresh water in a 2:3 ratio, without ill effect. The French physician Alain Bombard survived an ocean crossing in a small Zodiak rubber boat using mainly raw fish meat, which contains about 40 percent water (like most living tissues), as well as small amounts of seawater and other provisions harvested from the ocean. Naturally, the veracity of his findings was challenged, but an alternative explanation to Bombard's survival was not given. In Kon-Tiki, Thor Heyerdahl reported drinking seawater mixed with fresh in a 40/60% ratio. A few years later another adventurer named William Willis claimed to have drunk two cups of seawater and one cup of fresh per day for 70 days without ill effect when he lost part of his water supply.[12]
Most modern ocean-going vessels create drinkable (potable) water from seawater using desalination processes such as vacuum distillation or multi-stage flash distillation in an evaporator, or more recently by reverse osmosis. However these processes are energy intensive, and most were not usually available during the Age of Sail. Larger sailing warships with large crews, such as Nelson's HMS Victory were fitted with distilling apparatus in their galleys.[13]
Other land animals and marine animals such as fish, whales, and penguins can adapt to a high saline habitat. For example, the desert rat can survive by drinking seawater because its kidney can concentrate sodium far more efficiently than the human kidney.
Properties
The thermal conductivity of seawater is 0.6 W/mK at 25 degC and a salinity of 35 g/kg.[14] The thermal conductivity decreases with increasing salinity and increases with increasing temperature.[15]
See also
Footnotes
- ^ "U.S. Office of Naval Research Ocean, Water: Temperature". http://www.onr.navy.mil/Focus/ocean/water/temp3.htm.
- ^ Sylte, Gudrun Urd (May 24, 2010). "Den aller kaldaste havstraumen" (in Norwegian). forskning.no. http://www.forskning.no/artikler/2010/mai/250690. Retrieved May 24, 2010.
- ^ "World Ocean Atlas 2005". NOAA. http://www.nodc.noaa.gov/OC5/WOA05/pr_woa05.html. Retrieved 17 October 2010.
- ^ a b Gale, Thomson. "Ocean Chemical Processes". http://www.waterencyclopedia.com/Mi-Oc/Ocean-Chemical-Processes.html. Retrieved December 2, 2006.
- ^ a b Pinet, Paul R. (1996). 'Invitation to Oceanography. St. Paul: West Publishing Company. pp. 126, 134–135. ISBN 9780314063397.
- ^ C. Michael Hogan. 2010. Calcium. eds. A.Jorgensen, C. Cleveland. Encyclopedia of Earth. National Council for Science and the Environment.
- ^ DOE (1994). "5". In A.G. Dickson & C. Goyet. Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water. 2. ORNL/CDIAC-74. http://cdiac.esd.ornl.gov/ftp/cdiac74/chapter5.pdf.
- ^ Pinet 1996, p. 133
- ^ "Ask A Scientist". Biology Archive. http://www.newton.dep.anl.gov/askasci/bio99/bio99416.htm.
- ^ "Chapter 29 Shipboard Medicine". http://www.bordeninstitute.army.mil/published_volumes/harshEnv2/HE2ch29.pdf. Retrieved 17 October 2010.
- ^ Etzion and Yagil; Yagil, R (1987;86(1)). "Metabolic effects in rats drinking increasing concentrations of seawater.". Comp Biochem Physiol A. 86 (1): 49–55. doi:10.1016/0300-9629(87)90275-1. PMID 2881655.
- ^ King, Dean (2004). Skeletons on the Zahara: a true story of survival. New York: Back Bay Books. p. 74. ISBN 9780316159357.
- ^ Rippon, Commander P.M., RN (1998). The evolution of engineering in the Royal Navy. Vol 1: 1827-1939. Spellmount. pp. 78–79. ISBN 0-946771-55-3.
- ^ "Desalination and Water Treatment". aDepartment of Mechanical Engineering, Massachusetts Institute of Technology. April 2010. http://web.mit.edu/lienhard/www/Thermophysical_properties_of_seawater-DWT-16-354-2010.pdf. Retrieved 17 October 2010.
- ^ "Thermal conductivity of seawater and its concentrates". http://twt.mpei.ac.ru/tthb/2/Tab-5-5-13-2-Ther-Cond-Seawater.html. Retrieved 17 October 2010.
External links
Categories:- Aquatic ecology
- Chemical oceanography
- Liquid water
- Physical oceanography
Wikimedia Foundation. 2010.