- Membrane technology
-
The membrane technology covers all process engineering measures for the transport of substances between two fractions with the help of permeable membranes. That means in general mechanical separation process for separation of gaseous or liquid streams using membrane technology.
Contents
Applications
The particular advantage of membrane separation processes is that it operate without heating and thus are energetically usually lower than conventional thermal separation processes (distillation, Sublimation or crystallization). This separation process is purely physical and, thanks to its gentle separation, the use of both fractions (permeate and retentate) is possible. Therefore, the cold separation by membrane processes has been established particularly in the food technology, the biotechnology and pharmacy industrie. Furthermore, with the help of membrane separations realizeable that with thermal processes are not possible. For example, because azeotropics or isomorphics crystallization making a separation by distillation or recrystallization impossible. Depending on the type of membrane, the selective separation of certain individual substances or substance mixtures is possible. Important technical applications include drinking water by reverse osmosis (worldwide approximately 7 million cubic meters annually), filtrations in the food industry, the recovery of organic vapors such as gasoline vapor recovery and the electrolysis for chlorine production. But also in wastewater treatment, the membrane technology is becoming increasingly important. With the help of UF and MF (Ultra-/Mikrofiltration) it is possible to remove particles, colloids and macromolecules, so that wastewater can be disinfected in this way. This is needed if wastewater is discharged into sensitive outfalls, or in swimming lakes.
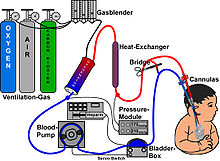
About half of the market has applications in medicine. As an artificial kidney to remove toxic substances by hemodialysis and as artificial lung for bubble-free supply of oxygen in the blood. Also the importance of membrane technology is growing in the field of environmental protection (NanoMemPro IPPC Database). Even in modern energy recovery techniques membranes are increasingly used, for example in the fuel cell or the osmotic power plant.
Mass transfer
For the mass transfer at the membrane, two basic models can be distinguished: the solution-diffusion model and the hydrodynamic model. In real membranes, these two transport mechanisms certainly occur side by side, especially during the ultrafiltration.
Solution-diffusion model
The transport is done only by diffusion. The component that need to be transported must be first dissolved in the membrane. This principle is more important for dense membranes without real Pores such as those used for reverse osmosis and in a fuel cell. During the filtration process is formed on the membrane a boundary layer. This concentration gradient is created by molecules which can not pass through the membrane. This effect is referred as concentration polarization. It occurs during the filtration and leads to a reduced transmembrane flow (flux). The concentration polarization is in principle reversible by cleaning the membrane and the initial flux can be almost restored. Also the use of a tangential flow to the membrane (cross-flow filtration) minimizes concentration polarization.
Hydrodynamic model
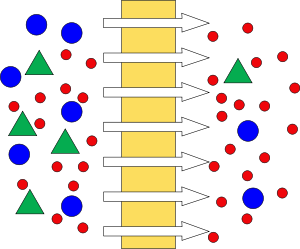
Transport through pores - in the simplest case of pure transport convectively. This requires the size of the Pores to be smaller than the diameter of the to separate components. Membranes, which function according to this principle are used mainly in micro- and ultrafiltration. They are used to separate macromolecules from solutions, colloids from a dispersion or remove bacteria. During this process the not passing particles or molecules are forming on the membrane a more or less a pulpy mass (filter cake). This hampered by the blockage of the membrane the filtration. By the so-called cross-flow method (cross-flow filtration) this can be reduced. Here, the liquid to be filtered flows along the front of the membrane and is separated by the pressure difference between the front and back of the fractions into retentate (the flowing concentrate) and permeate (filtrate). This creates a shear stress that cracks the filter cake and lower the formation of fouling.
Membrane operationes
According to driving force of the operation it is possible to distinguish:
- pressure driven operations
- concentration driven operations
- operations in electric potential gradient
- electrodialysis
- membrane electrolysis
- electrophoresis
- operations in temperature gradient
- membrane distillation
Membrane shapes and flow geometries
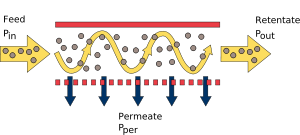
There are two main flow configurations of membrane processes: cross-flow and dead-end filtrations. In cross-flow filtration the feed flow is tangential to the surface of membrane, retentate is removed from the same side further downstream, whereas the permeate flow is tracked on the other side. In dead-end filtration the direction of the fluid flow is normal to the membrane surface. Both flow geometries offer some advantages and disadvantages. The dead-end membranes are relatively easy to fabricate which reduces the cost of the separation process. The dead-end membrane separation process is easy to implement and the process is usually cheaper than cross-flow membrane filtration. The dead-end filtration process is usually a batch-type process, where the filtering solution is loaded (or slowly fed) into membrane device, which then allows passage of some particles subject to the driving force. The main disadvantage of a dead end filtration is the extensive membrane fouling and concentration polarization. The fouling is usually induced faster at the higher driving forces. Membrane fouling and particle retention in a feed solution also builds up a concentration gradients and particle backflow (concentration polarization). The tangential flow devices are more cost and labor intensive, but they are less susceptible to fouling due to the sweeping effects and high shear rates of the passing flow. The most commonly used synthetic membrane devices (modules) are flat plates, spiral wounds, and hollow fibers.
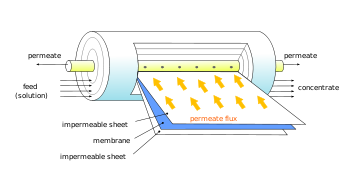
Flat plates are usually constructed as circular thin flat membrane surfaces to be used in dead-end geometry modules. Spiral wounds are constructed from similar flat membranes but in a form of a “pocket” containing two membrane sheets separated by a highly porous support plate.[1] Several such pockets are then wound around a tube to create a tangential flow geometry and to reduce membrane fouling. Hollow fiber modules consist of an assembly of self-supporting fibers with a dense skin separation layers, and more open matrix helping to withstand pressure gradients and maintain structural integrity.[1] The hollow fiber modules can contain up to 10,000 fibers ranging from 200 to 2500 μm in diameter; The main advantage of hollow fiber modules is very large surface area within an enclosed volume, increasing the efficiency of the separation process.
Membrane performance and governing equations
The selection of synthetic membranes for a targeted separation process is usually based on few requirements. Membranes have to provide enough mass transfer area to process large amounts of feed stream. The selected membrane has to have high selectivity (rejection) properties for certain particles; it has to resist fouling and to have high mechanical stability. It also needs to be reproducible and to have low manufacturing costs. The main modeling equation for the dead-end filtration at constant pressure drop is represented by Darcy’s law:[1]

where Vp and Q are the volume of the permeate and its volumetric flow rate respectively (proportional to same characteristics of the feed flow), μ is dynamic viscosity of permeating fluid, A is membrane area, Rm and R are the respective resistances of membrane and growing deposit of the foulants. Rm can be interpreted as a membrane resistance to the solvent (water) permeation. This resistance is a membrane intrinsic property and expected to be fairly constant and independent of the driving force, Δp. R is related to the type of membrane foulant, its concentration in the filtering solution, and the nature of foulant-membrane interactions. Darcy’s law allows to calculate the membrane area for a targeted separation at given conditions. The solute sieving coefficient is defined by the equation:[1]

where Cf and Cp are the solute concentrations in feed and permeate respectively. Hydraulic permeability is defined as the inverse of resistance and is represented by the equation:[1]

where J is the permeate flux which is the volumetric flow rate per unit of membrane area. The solute sieving coefficient and hydraulic permeability allow the quick assessment of the synthetic membrane performance.
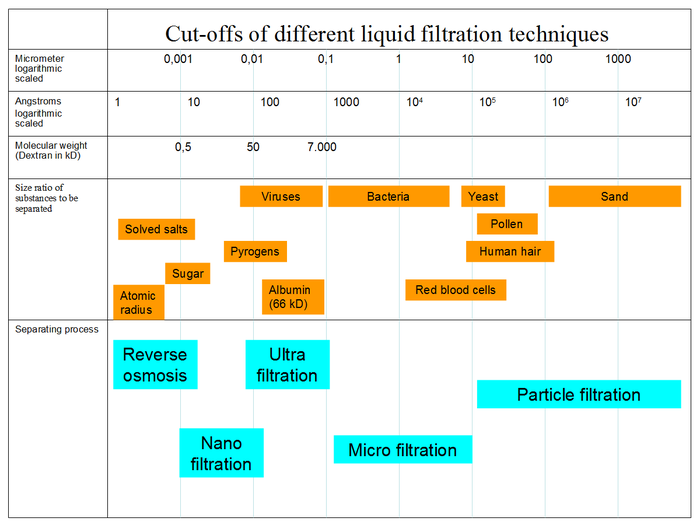
Membrane separation processes
Membrane separation processes have very important role in separation industry. Nevertheless, they were not considered technically important until mid-1970. Membrane separation processes differ based on separation mechanisms and size of the separated particles. The widely used membrane processes include microfiltration, ultrafiltration, nanofiltration, reverse osmosis, electrolysis, dialysis, electrodialysis, gas separation, vapor permeation, pervaporation, membrane distillation, and membrane contactors.[2] All processes except for pervaporation involve no phase change. All processes except (electro)dialysis are pressure driven. Microfltration and ultrafiltration is widely used in food and beverage processing (beer microfiltration, apple juice ultrafiltration), biotechnological applications and pharmaceutical industry (antibiotic production, protein purification), water purification and wastewater treatment, microelectronics industry, and others. Nanofiltration and reverse osmosis membranes are mainly used for water purification purposes. Dense membranes are utilized for gas separations (removal of CO2 from natural gas, separating N2 from air, organic vapor removal from air or nitrogen stream) and sometimes in membrane distillation. The later process helps in separating of azeotropic compositions reducing the costs of distillation processes.
See also
Notes
References
- Osada, Y., Nakagawa, T., Membrane Science and Technology, New York: Marcel Dekker, Inc,1992.
- Zeman, Leos J., Zydney, Andrew L., Microfiltration and Ultrafitration, Principles and Applications., New York: Marcel Dekker, Inc,1996.
- Mulder M., Basic Principles of Membrane Technology, Kluwer Academic Publishers, Netherlands, 1996.
- Jornitz, Maik W., Sterile Filtration, Springer, Germany, 2006
- Van Reis R., Zydney A. Bioprocess membrane technology. J Mem Sci. 297(2007): 16-50.
- Templin T., Johnston D., Singh V., Tumbleson M.E., Belyea R.L. Rausch K.D. Membrane separation of solids from corn processing streams. Biores Tech. 97(2006): 1536-1545.
- Ripperger S., Schulz G. Microporous membranes in biotechnical applications. Bioprocess Eng. 1(1986): 43-49.
- Thomas Melin, Robert Rautenbach, Membranverfahren, Springer, Germany, 2007, ISBN 3-540-00071-2.
- Munir Cheryan, Handbuch Ultrafiltration, Behr, 1990, ISBN 3-925673-87-3.
- Eberhard Staude, Membranen und Membranprozesse, VCH, 1992, ISBN 3-527-28041-3.
Categories:- Filtration
- Membrane technology
- Chemical engineering
- Unit operations
Wikimedia Foundation. 2010.